Page 1

www.ActiveForever.com
Continuous Passive Motion Device (CPM)
Operator Manual
Phoenix Model 1800 Knee CPM
Phoenix Model 1850 Knee CPM
DC2480 Knee CPM
Page 2

www.ActiveForever.com
FOREWARD
This manual has been written for operators of the Furniss Corporation Continuous Passive Motion (CPM) Therapy Unit.
It contains general information for operation, precautionary instructions and maintenance recommendations. In order to obtain
maximum life and efficiency from your Furniss Corp CPM Unit, and to assist in the proper operation of the unit, read and
understand this manual thoroughly. It is recommended that all clinicians and support personnel become familiar with the
operating procedures, as well as the indications, contraindications, warnings and precautions prior to administering patient
treatment.
Product Description
Continuous Passive Motion is a postoperative procedure designed to aid in patient recovery after joint surgery, soft tissue
surgical procedure or trauma. The treatment method has been practiced for more than 30 years. This unit is typically used
postoperatively for total knee replacement and Anterior Cruciate Ligament (ACL) repairs.
After extensive joint surgery patient attempts at joint flexion can cause considerable pain, dissuading the patient from effective
joint motion. Failure to achieve proper rehabilitative flexion can lead to stiffening of the tissue around the joint. Eventually, this
condition may cause intra-articular adhesions (formation of scar tissue) and extra-articular contractures (shortening of a muscle
or tendon), resulting in a limited range of motion for the joint.
Passive range of motion moves the joint gradually and slowly without the use of the patient’s muscles. The device is applied
post-operatively and can be used in both inpatient and outpatient therapy regimens. The physician will prescribe usage
instructions, including the speed of the machine, the duration of usage, amount of motion and the rate of motion increase.
Various studies have concluded that patients using a CPM machine following surgery generally reduce the risk of complications
and require less pain medication than those who do not use the CPM device after the same type of surgery.
About the Manufacturer
As a US based medical manufacturer, The Furniss Corporation builds Continuous Passive Motion (CPM) devices for the upper
and lower extremities. The durable, user friendly devices are built to withstand the rigorous hospital and home care
environment. Furniss Corp also manufactures patient softgoods and offers a comprehensive technical service and repair
center. Expect same-day shipping, immediate access to educated product professionals and strong product lines with
exceptional performance. Visit the website at KneeCPM.com to experience the interactive tour.
Page 3

www.ActiveForever.com
PRECAUTIONARY INSTRUCTIONS
CAUTION
Read, understand and practice the precautionary instructions found in this manual before operating or using the unit.
Know the limitations and hazards associated with using the device. Observe any and all precautionary and operational
labels placed on the unit.
Do not use the Furniss Corp CPM device outdoors or on wet surfaces. Use only on firm, flat, level surfaces to ensure
stability of the unit while in operation.
Extreme caution should be taken when in use with or around children.
Use the CPM device only for its intended purpose as described in this manual.
Turn power switch off before unplugging unit from its power source.
Do not touch any exposed or uninsulated wires or terminals unless the transformer has been unplugged.
Do not use the cord to unplug the power cord from the power source. Grasp at the power cord base. Use a surge protector
to further safeguard the unit.
Transport and store the device in temperatures between –40 degrees Celsius to +70 degrees Celsius to prevent damage
to the unit or its components. Condensation could result and damage the Furniss Corp CPM if unit is subjected to periods
of low temperatures followed by periods of high temperatures. Additional environmental conditions include: relative
humidity range within 10% to 100%. Atmospheric pressure range within 500 to 1060 hPa.
Use care when carrying, transporting or storing the device to prevent damage to the unit from dropping or improper
transport and storage methods.
WARNING
U.S. Federal law restricts this device to sale by, or on the order of, a physician or licensed practitioner.
Make certain that the unit is electrically grounded by connecting only to a grounded electrical service receptacle conform-
ing to the applicable national and local electrical codes.
Keep obstructions such as hair, loose clothing, bedding, pets, fingers and toes away from the ballscrew assembly and
hinge components of the unit.
The Furniss Corp CPM device must be completely visible at all times during use. Never cover the unit with bedding or any
other means of concealment while in operation.
Do not use the Furniss Corp CPM device while smoking or around open flame.
Equipment no suitable for use in presence of a flammable anesthetic mixture with air, oxygen or nitrous oxide.
Precautionary measures should be taken when any type of liquid comes in contact with an electrical apparatus.
Always turn off and unplug unit from electrical source before servicing or cleaning. Failure to do so could result in electrical
shock or personal injury.
Handle the unit only when the unit is dry and hands are dry to prevent electrical shock.
Unconscious patients or patients under heavy influence of medication must be constantly attended and monitored while
the device is in use.
Page 4
www.ActiveForever.com
UNPACKING YOUR CPM
When receiving your Furniss Corp CPM, examine the container and unit and report any substantial damage to the shipper. Do
not discard your shipping container - it provides required protection for your unit during shipping and must be used when
returning your unit for any warranty service. Unapproved or badly damaged containers will be replaced and billed accordingly.
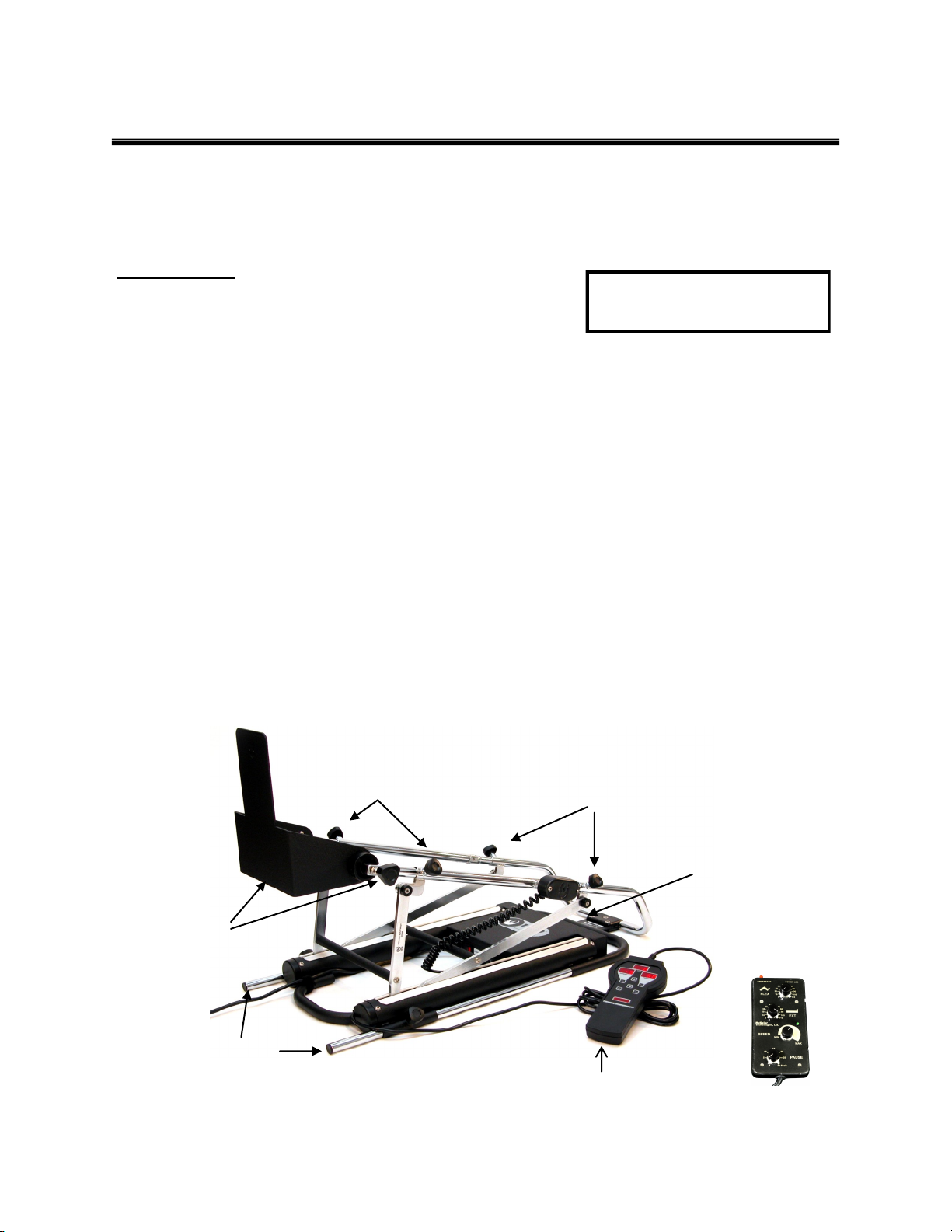
Container Contents
> CPM Cradle Assembly with Control Pendant
> Foot Cradle Assembly
> Two (2) Bed Stabilizer Rods
> One (1) Set of 1828-1 Patient Softgoods
> Operator Manual, Quick Reference Guide, CPM Model Specification Sheet
Remove the Knee CPM from the container and slide the Foot Cradle Assembly out.
Loosen the large knobs on the Foot Cradle Assembly and position in the upright position. Tighten knobs.
CPM Shipping Carton
Part No. Skit 1824
Foot Cradle Assembly
Foot Cradle
Adjustment Knobs (2)
Bed Stabilizer Rods (2)
Thigh Length
Adjustment Knobs (2)
Control Box with
Circuitry
Analog Control
Pendant
Digital Control Pendant
Page 5
www.ActiveForever.com
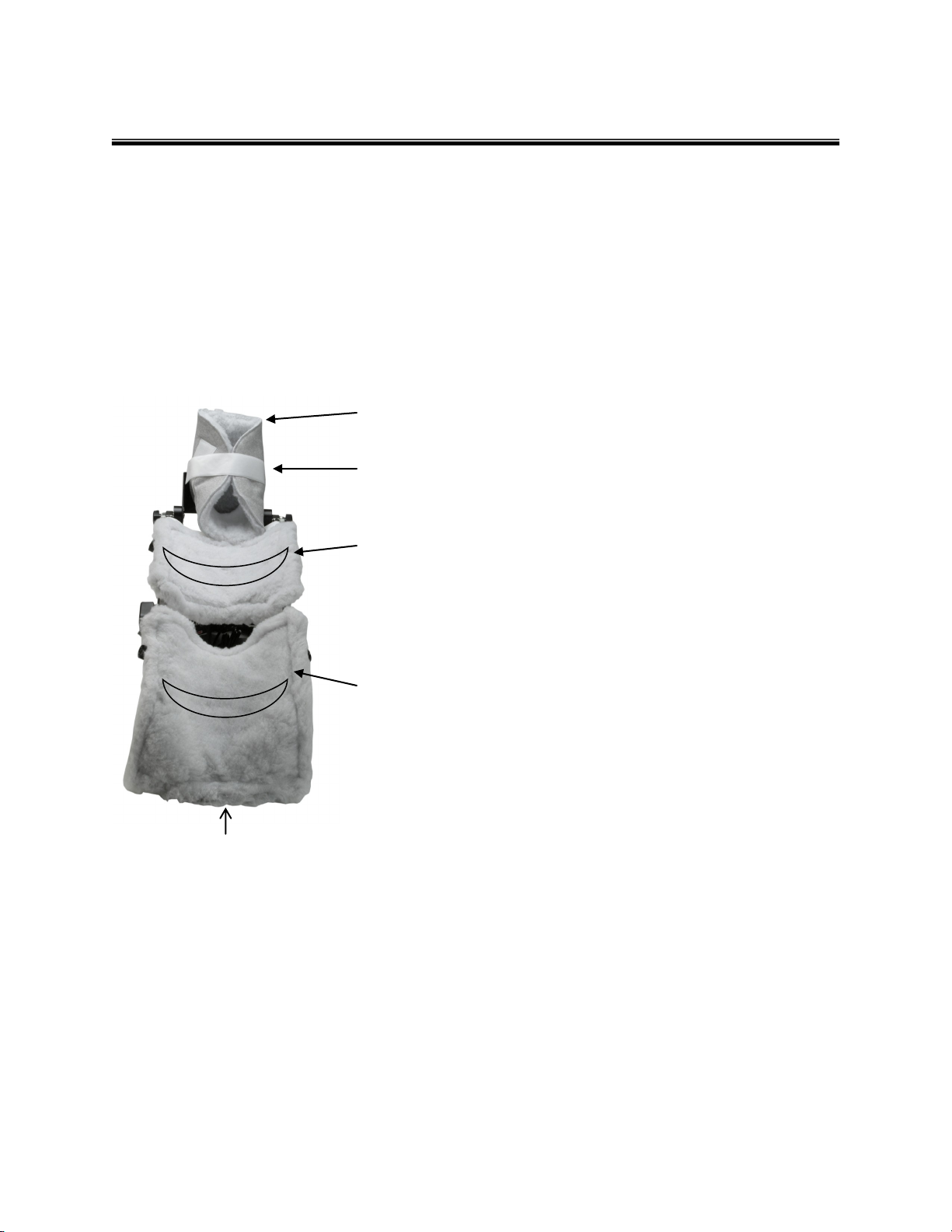
SOFTGOODS APPLICATION
Furniss Softgoods 1828-1 are designed specifically for use with Furniss Knee CPM devices. Softgoods provide a 'troughing'
effect allowing proper alignment of the limb and a truer fit overall. Constructed with the highest quality materials. Furniss
Softgoods provide superior comfort and support for the patient.
Each Patient Softgoods kit includes five (5) pads total:
1 - Foot Boot
1 - Calf Pad
1 - Thigh Pad
2 - Auxiliary Straps
Slide the pocket of the foot boot over the end of the foot plate and secure to the
Velcro.
Secure ankle support straps on the foot boot to the foot assembly tubes on both
sides.
The calf pad is the smaller of the two square-shaped pads. Position the calf pad so
TROUGH
TROUGH
that the cut-out is facing the thigh cradle. Peel the velcro strapping from the back of
the pad, loop over the cradle bar of the unit and secure to the back of the softgood.
Be sure not to pull the softgood tight, forming a trough to allow for proper alignment.
The thigh pad is the larger of the two square-shaped pads. Position the thigh pad so
that the cut-out is facing the calf pad (the cut-outs will form a 'diamond' shape).
Begin with the tab Velcro at the very end of the thigh pad. Secure this piece to the
bottom of the thigh block**. Peel the rest of the Velcro strapping from the back of
the pad, loop over the cradle bar of the unit and secure to the back of the softgood.
Be sure to 'trough' the softgood, do not pull the softgood tight.
The two (2) auxiliary straps may be used to secure the thigh and/or calf (do not secure too tightly—leave room for shifting).
Softgoods are intended for single patient use only. Should the pads become soiled during use, spot clean by hand with mild
detergent and warm water.
Furniss Softgoods are designed specifically for use with the Knee CPM Series. Use of any other brand of CPM Softgoods may
adversely affect performance of the Knee CPM device and result in discomfort or injury to the patient.
Reorder number for the Furniss Softgoods: SKIT 1828-1
** thigh block
Page 6
www.ActiveForever.com
PATIENT SETUP
Correct measurement and adjustments are required to ensure patient comfort and compliance and to achieve desired range of
motion. Check that the unit is at 0 degrees during patient setup.
Note: Use only on a stable surface.
An unstable surface could result in injury to patient and damage to the device.
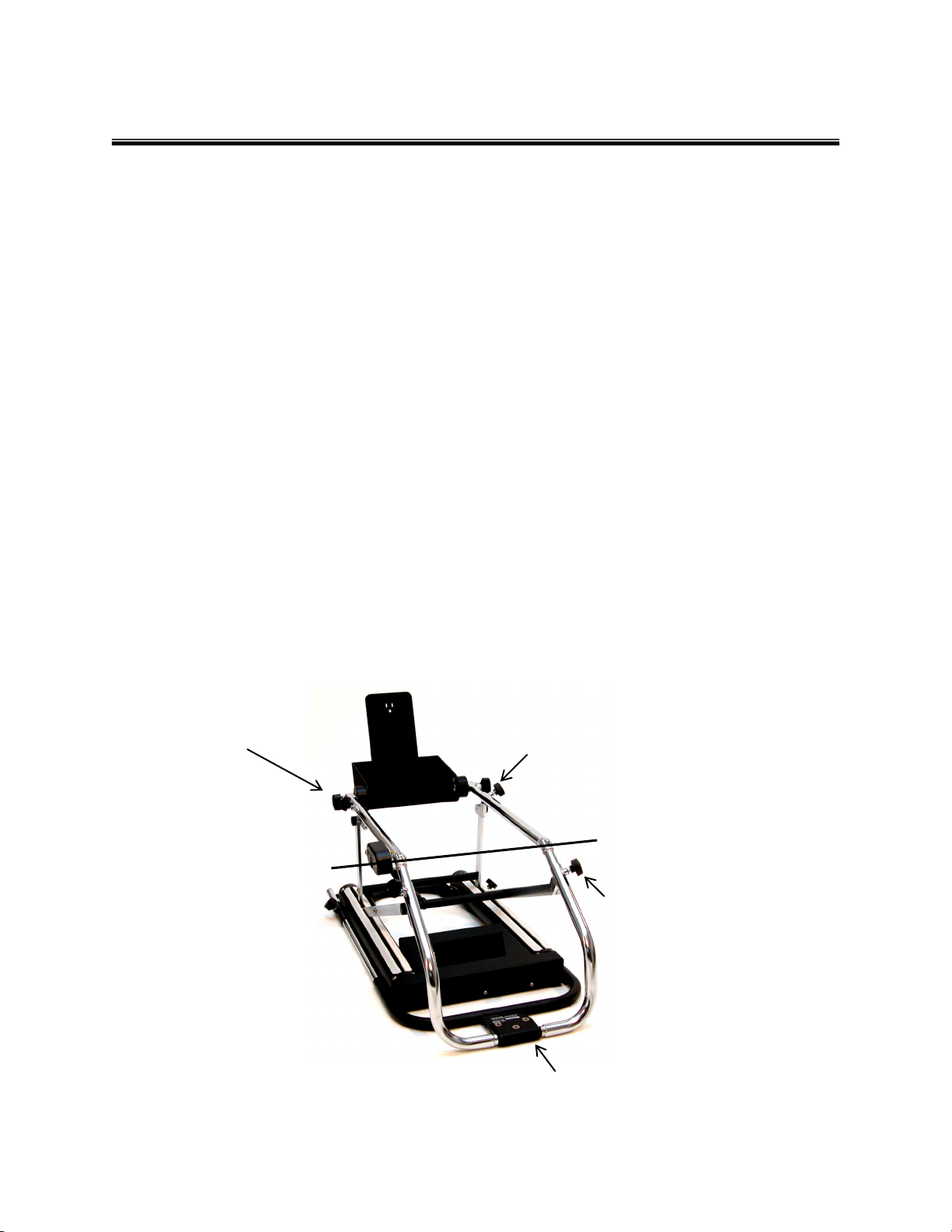
FITTING THE PATIENT
Determine the length of the patient’s femur by measuring from the greater trochanter (hip joint) to the center or joint line of
the knee.
Transfer this measurement to the thigh cradle beginning approximately 1.5 to 2 inches away from the hip pivot assembly
measuring to the knee axis of the unit. Adjust the thigh cradle to match this measurement by loosening the thigh
adjustment knobs and sliding the thigh cradle to the proper length. Note: The DC2480 maximum cradle adjustment is 3
inches. Do not extended beyond this point. If patient’s thigh length exceeds these measurements, simply increase the
beginning point of measurement to 4 to 6 inches away from the hip pivot assembly.
Loosen the calf cradle adjustment knobs and extend the foot assembly. Do not remove this assembly -- only slide far
enough for patient placement. Position the patient's leg in the unit, with softgoods in place.
Slide the foot assembly toward patient accordingly leaving one half inch gap between the patient’s foot and the foot plate.
Tighten the calf cradle adjustment knobs securely.
The foot assembly may be adjusted in plantar flex or dorsi flex positions. Loosen the adjustment knobs on the foot
assembly, adjust the foot plate to the desired positioning and securely tighten the adjustment knobs.
Remember: The goal is to align the knee axis of the patient with the knee pivot axis of the CPM
Foot assembly adjustment knobs (2)
Knee pivot axis of unit
Calf adjustment knobs (2)
Thigh adjustment knobs (2)
Hip pivot assembly
Page 7

www.ActiveForever.com
PATIENT SET UP
BED STABILIZING SYSTEM
Bed stabilizer rods are included in the base of each Furniss Knee CPM. This system, in conjunction with the non-skid pad
located on the bottom of the control box, effectively prevents migration or "movement" of the unit while on a patient.
Loosen the base knobs, extend the rods to the appropriate length and tighten the knobs securely.
Stabilize the rods against a solid barrier such as a foot board.
PEDIATRIC AND PETITE PATIENT ADJUSTMENT
The Furniss Knee CPM can adjust easily to accommodate pediatric and petite patients.
Loosen the calf adjustment knobs and slide the foot assembly out of the cradle.
Loosen the foot assembly adjustment knobs (do not remove).
Rotate both foot tubes 180 degrees so that they are facing the opposite direction.
Tighten the foot assembly adjustment knobs.
Slide cradle back into the device and secure with
the calf adjustment knobs.
Note: You may apply a strip of adhesive-backed
loop Velcro to this side of the footplate for added
stability of the foot boot softgood.
Bed stabilizer rods (2)
Base knobs (2)
Page 8

www.ActiveForever.com
OPERATING THE PHOENIX MODEL 1850 AND DC2480 CPM
POWER ON AND OFF
The ON/OFF switch is located at the base of the black control box. When the unit is powered ON it will beep one (1) time and
the hand-held control pendant will illuminate. This indicates that the unit is ready for use.
PHOENIX MODEL 1850 KNEE CPM CONTROL PENDANT
The Model 1850 control pendant includes of the following functionalities:
Flexion: 0 to 110 degrees
Extension: -5 to 105 degrees
Speed: 1 degree per second at lowest setting, 3 degrees per second at highest setting
Pause: 0 to 30 Seconds*
Patient Compliance Meter
Patient Lock Out Switch
DC2480 KNEE CPM CONTROL PENDANT
The DC2480 control pendant includes of the following functionalities:
Flexion: 0 to 120 degrees
Extension: -10 to 105 degrees
Speed: 1 degree per second at lowest setting, 3 degrees per second at highest setting
Pause: 0 to 30 Seconds*
Patient Compliance Meter
Patient Lock Out Switch
To set Flexion range: Depress the FLX button and the up or down arrow simultaneously.
To set Extension range: Depress the EXT button and the up or down arrow simultaneously.
A 5 degree difference between flexion and extension is required for normal operation.
Should the angle settings be too close the incorrect setting on the pendant will flash.
To set Speed: Depress the Speed button and the up or down arrow simultaneously.
When you depress the Speed button, the setting will appear in the ROM window.
To set *Pause: Depress the Pause button and the up or down arrow simultaneously.
When you depress the Pause button, the setting will appear in the ROM window.
Patient Compliance Meter: The patient compliance meter monitors patient usage and tracks in tenths of an hour readings.
Example: 25.4 reading = 25 hours and 24 minutes.
- Meter is displayed in the Flexion window
- The meter is read by depressing the FLX and SPD buttons simultaneously
- Clear meter by depressing the EXT, FLX, SPD and Pause buttons simultaneously.
Note: The Start/Stop button must be pressed in order to lock in setting changes.
Page 9

www.ActiveForever.com
DIGITAL CONTROL PENDANT OVERVIEW
Range of Motion (ROM) window
Extension window
Extension button
*Pause button
Flexion window
Flexion button
Speed button
Patient must have access to the
hand-held control pendant at all times.
The Stop/Start button allows patient to
stop the unit as needed. As a safety
feature, the unit will travel in the opposite
direction when the button is depressed
again.
Patient Lock-Out function:
The lock-out switch is located on the back of the
control pendant under the plastic door cover.
To prevent inadvertent changes to pendant settings, simply slide
the switch to the locked position. The ROM window will read
'LOC' should attempt be made to adjust settings when this is in
place. The stop/start button functionality remains the same.
*Pause option:
Phoenix Models are set to pause at the end of flexion
and extension cycles. This setting can be changed to
pause the CPM device in extension only.
Page 10

www.ActiveForever.com
OPERATING THE PHOENIX MODEL 1800
Power On and Off
The ON/OFF switch is located at the base of the black control box. When the unit is powered ON it will beep 3 times and the
green power indicator light on the hand-held control pendant will illuminate. This indicates that the unit is ready for use.
Phoenix Model 1800 Knee CPM Control Pendant
The control pendant consists of four (4) control knobs:
1. Flexion: 10 to 110 degrees
2. Extension: -5 to 95 degrees
3. Speed: 1 degree per second at lowest setting, 3 degrees per second at highest setting
4. Pause: 0 to 30 Seconds*
> To change the flexion and extension settings, simply turn the control knobs to the
desired range of motion.
Note: A 15 degree difference between flexion and extension is required for normal operation. Should the angle settings be
close or overlap, the unit will stop and a continuous beeping alert will sound until the settings are corrected.
> Set speed and pause by turning the corresponding knob.
> Press the red button at the top of the pendant to begin cycle or to stop the unit.
> Close the cover to protect analog dials, and secure with the Velcro strap.
Patient must have access to hand-held
control pendant at all times.
The Stop/Start button located at the top of
the control pendant allows patient to stop the unit as
needed. As a safety feature, the unit will travel in the
opposite direction when the button is depressed again.
*Pause option:
Phoenix Models are set to pause at the end of flexion
and extension cycles. This setting can be changed to
pause the CPM device in extension only.
Power indicator light
Page 11

www.ActiveForever.com
MAINTENANCE
Recommended Tools
Small Phillips Screwdriver 1/8 T Handle Allen Wrench Basic Volt Meter
Small Flat Head Screwdriver 3/32 T Handle Allen Wrench Light Building Grease
(front end cap roller bearing)
Phillips Screwdriver 9/64 T Handle Allen Wrench Red Loctite
(shoulder bolt/acorn nut/calf + thigh tube bolt)
Large Flat Head Screwdriver 5/32 T Handle Allen Wrench Blue Loctite
(strut support bolt/set screws on pulleys + couplings)
Small Needle Nose Pliers 3/4 Wrench
(pendant cable/knee pot strain relief)
Standard Pliers 5/64 Hex Key Allen Wrench
(set screws)
Vise Grips 7/16 Nut Driver
(foot plate)
Small Rubber Hammer Small Crescent Wrench Velcro Cleaner
After each use, clean the unit using a soft cloth dampened with water and a mild antibacterial detergent or sani-cloth
disinfecting wipe.
Pendant Care
The pendant is a vital component of the entire CPM device. Furniss Corp builds two types of pendants for the equipment
manufactured. The Phoenix Model 1850 and the DC2480 feature a digital pendant and the Phoenix Model 1800 maintains an
analog hand control.
Digital Pendant (Model 1850/ Model DC2480):
Inspect the overlay for any damage. Confirm operation of the pendant cable. Replace as needed.
Analog Pendant (Model 1800):
Fasten the Start/Stop switch tightly. Secure the control knobs. Verify operation of the pendant cable. Replace as needed.
Tighten All Fasteners
Ensure all of the knobs, screws, bolts and other fasteners are secure to maintain operational use. It is recommend that these
parts are checked each time a unit is placed on a patient to guarantee peak performance. A suggested Locktite product assists
to secure the fasteners. Contact the Technical Service Department for recommendations on which type of Locktite to use on
certain fasteners. Toll free 800.273.5233.
Purple Loctite
(thigh block screws/small allen bolt in foot plate)
Cleaning Supplies for adhesive removal / Goo Gone
100% Clear Silicone Adhesive
(Order # adhesive)
Page 12

www.ActiveForever.com
MAINTENANCE
Track Seal Maintenance
Track seals serve to protect each ball screw component. If the track seal is ripped or torn, it must be replaced. A damaged
track seal allows large particles to enter the ball screw assembly, thus causing the unit to not operate properly. Remove the dry
adhesive from the unit before continuing. Replace the track seal following instructions provided with the part. The best
adhesive to use is a product that is 100% silicone, which is available for purchase from Furniss Corp. Do Not Use any kind of a
super glue or cement paste.
Ball Screw Assembly
The ball screw assembly assists the leg carriage to progress between different degrees of flexion and extension. This part of
the unit is in constant motion and it is very important that the entire assembly is properly maintained.
Ensure the screw is properly lubricated. The ball screw assembly should have a wet appearance. Excessive lubricant will
affect the performance. Furniss Corp recommends white building grease for optimum function. A lithium based lubricant is
also acceptable. We do not recommend any type of spray lubricant or grease – including WD40, or a spray silicone
product. Do not use heavy grease for lubrication.
Keep the ball screw assembly free of debris. Refer to previous track seal section for additional prevention details.
Use patient softgood set item #1828-1 for these units. Furniss Corp softgoods have been specifically designed to properly
fit the CPM unit. By using this set it will eliminate any opportunity for large pieces of debris to enter the ball screw
assembly. Please call our Client Services Department at 800.273.5233 to order the softgood pads (Item# 1828-1).
Carbon Removal from the Motor
Overtime carbon dust will be generated by the motor. The build-up of this residue can affect the efficiency of the motor. With
continuous use, the carbon should be removed from the motor every three months to extend the life of the part. Follow the
steps below to clean carbon from the motor.
Remove the motor and gearbox from the CPM unit.
Loosen the two screws at the top of the motor.
Blow compressed air through that opening which will remove the dust.
Summary
These are just a few maintenance suggestions to assist in the performance and longevity of the CPM units. Contact the
Technical Service Department before purchasing any replacement parts for your CPM unit. Furniss Corp is the only supplier for
original equipment parts. Should the CPM unit require service, please do not hesitate to contact us. As always, we are here to
answer any questions and help with any issues regarding the CPM units.
Page 13

A S im p le P re v en ta tiv e M a in te n an ce
P ro c ed u re to C le ar th e C a rb o n from th e
P h oe nix M o to r
P h il li p s H e ad S c rew s
Blow a ir thro ug h th e g a p s
A ll e n S c r e w s
1 2 3
To ll F re e 80 0 .2 7 3.5 2 3 3 6 1 4 .8 7 1 .1 47 0 F a x : 6 1 4 .8 7 1 .1 4 7 0
www.ActiveForever.com
MOTOR ONLY PREVENTIVE MAINTENANCE INSTRUCTIONS
TOOLS REQUIRED:
Phillips Head Screwdriver
9/64th Allen Wrench
Compressed Air
INSTRUCTIONS:
Turn the power to the unit off.
Unplug the motor from the circuit board.
Using the 9/64 Allen Wrench remove the allen screws that hold the Drive Assembly.
Remove the Drive Assembly.
Loosen the Phillips Head Screws enough to create a gap between the three (3) sections of the motor.
Caution: Do not remove the screws.
Blow enough air through the three (3) gaps to
clear the carbon build up.
Tighten the screws to secure the sections.
Return the Drive Assembly and test the unit.
Plug
Page 14
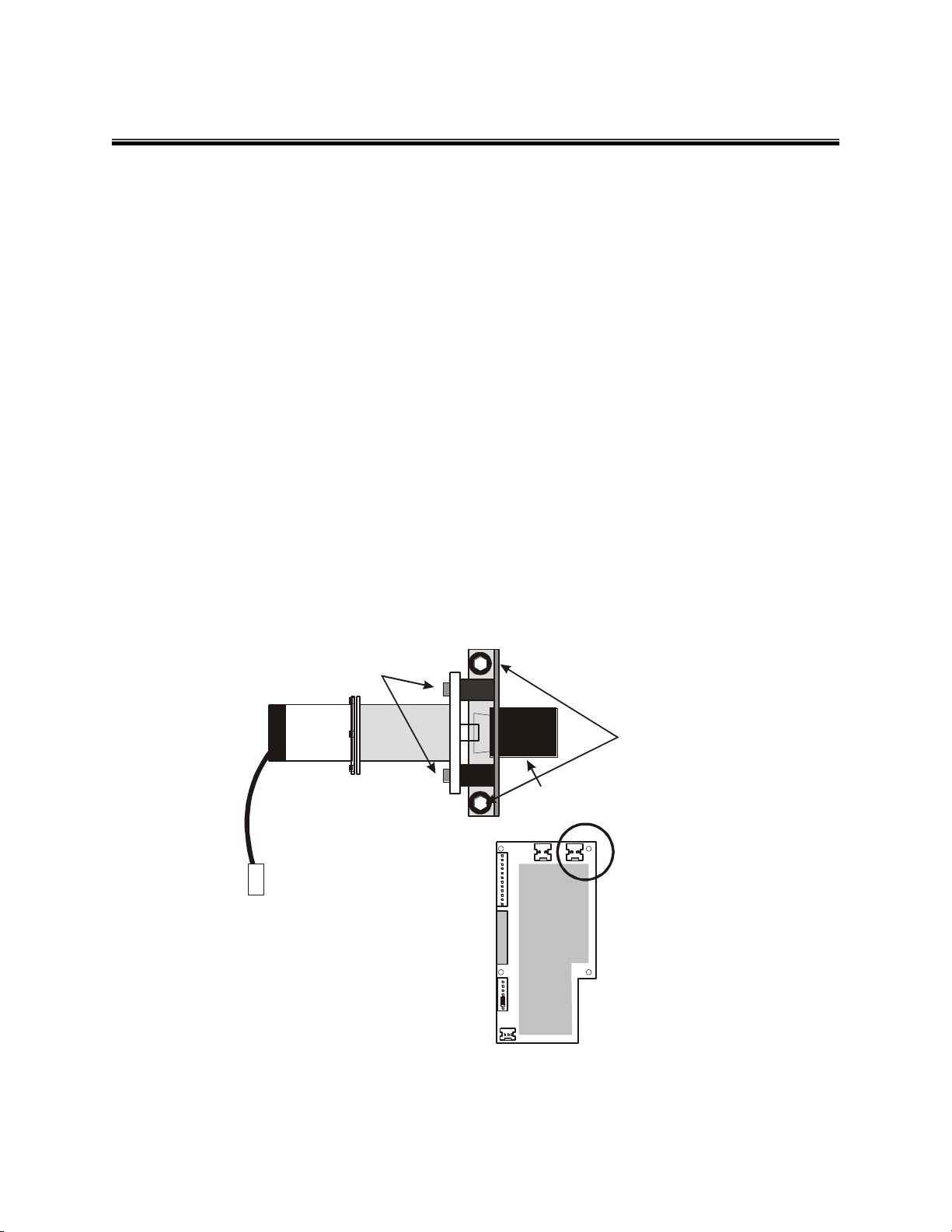
R e p la c in g the M oto r & G earb ox
To o ls r e q u ired :
Ph ill ip s H ead S crew dr iv e r, S m al l F la t H ea d
Sc rew d r ive r, 9 /6 4 t h A lle n W re n c h
1. T u rn th e P ow er S w itc h O FF.
2. R em ov e t h e C ontro l B o x C ov e r. ( 5 Ph ill ips
Sc rew s )
3. U np lu g the M oto r fr o m th e C i r cu it B o ard.
4. R em ov e t h e M oto r a n d g e a r b o x a s s e m bly u s i n g th e A lle n
W ren c h to u n s c r ew the t w o(2 ) s c r e w s f rom th e m o to r
m ou n tin g brack et.
5. U np lu g the g ea rb ox c o up li n g from th e ru bb er c o u p l e r.
6. R em ov e t h e m oto r m ou n t bracket b y rem ovin g t h e (4 )
A lle n sc rew s from the r u b b er b u s h in g s ..
7. A tta c h the N EW m o to r a n d g ea rb ox.
8. P lug t he c ou plin g in to t h e ru bb e r c o up le r.
9. S ecure t he m oto r m ountin g b r a c k e t w ith th e tw o (2 )
s c r e w s.
10 . Plug in th e m oto r t o th e C i r cu it B o ard.
11 . R e pla c e the t o p c o v e r.
R u b b e r C ou p le r
M o t or M oun t B rac ke t
A ll en S cre ws ( 2 )
R u b b e r B us hin g A ll e n S cre ws (4 )
P lu g
J1 J2
J3
J4
J6
Po wer Mo tor
Pe nda ntN/ACa libr ati on
Kn ee
Po ten tio me ter
J5
0 90 PA US E
To ll F re e 80 0 .2 7 3.5 23 3 6 1 4 . 8 7 1 .1 4 7 0 F a x : 6 1 4 .8 7 1 .1 4 7 0
1 9 0 3 LM
www.ActiveForever.com
MOTOR & GEARBOX REPLACEMENT INSTRUCTIONS
TOOLS REQUIRED:
Phillips Head Screwdriver
Small Flat Head Screwdriver
9/64th Allen Wrench
INSTRUCTIONS:
Turn the Power Switch OFF.
Remove the Control Box Cover. (5 Phillips Screws or 4 Phillips Screws for newer models)
Unplug the Motor from the Circuit Board.
Remove the Motor and gearbox assembly from the bottom cover using the Allen Wrench to unscrew the two (2) screws from
the motor mounting bracket.
Unplug the gearbox coupling from the rubber coupler.
Remove the motor mount bracket by removing the four (4) Allen screws from the rubber bushings.
Attach the NEW motor and gearbox. Plug the coupling into the rubber coupler.
Secure the motor mounting bracket with the two (2) screws. Plug in the motor to the Circuit Board. Replace the top cover.
Page 15

W H I T E W IR E
B L A C K W IRE
FR O M
P OW ER SU PP LY
TO C IRC UI T
B O AR D
R e p la c in g the P o w e r S u p ply
O N /O F F S W IT C H
G R OU ND
W IR E
(G RE E N )
To ll F re e 80 0 .2 7 3.5 23 3 6 1 4 . 8 7 1 .1 4 7 0 F a x : 6 1 4 .8 7 1 .1 4 7 0
www.ActiveForever.com
POWER SUPPLY REPLACEMENT INSTRUCTIONS
IMPORTANT NOTATIONS:
New power supply may be tested using a multi-meter. Ensure that wires do not touch while testing.
Verify that power supply is NOT PLUGGED IN during installation
TOOLS REQUIRED:
Reorder No. Skit 1511A
Pliers
Phillips Head Screwdriver
Large Flat Head Screwdriver
INSTRUCTIONS:
CAUTION: To avoid electric shock unplug the unit before beginning this procedure.
Turn the Power Switch OFF and unplug the unit from wall outlet.
Remove the Control Box Cover. (5 Phillips Screws or 4 Phillips Screws for newer models)
Unplug the white and black wires from the Power Supply side of the On/Off Switch. (see illustration)
Disconnect the green ground wire.
Using a flat head screw driver, get between the back side of the strain relief and the control box. Pry in an outward motion. The
strain relief will pull out.
Remove the power supply cord. Using pliers attach the strain
relief to the new power supply cord.
Insert the new power supply cord through the opening.
Replace the strain relief.
Plug in the white and black wires as indicated. (refer to illustration)
Attach the green ground wire.
Test the device for operation. Reinstall top cover.
Page 16

www.ActiveForever.com
TRACK SEAL REPLACEMENT INSTRUCTIONS
TOOLS REQUIRED:
Track Seal Kit — Reorder No. Skit 1707A
Adhesive (100% silicon) Reorder Code. ‘Adhesive’
Non-Abrasive Adhesive Remover
INSTRUCTIONS:
Remove the old track seals.
Clean off any adhesive residue from the old track seal. Surfaces must be cleaned thoroughly for the new track seal to adhere
properly. Clean with a quality, non-abrasive adhesive remover such as Goo-Gone.
Attach the track seals to the edge of the track opening by sliding the open side of the new track seal along the edge of the
opening.
Lift the top side of the new track seal and apply a bead of adhesive along the full length of the entire strip.
Ensure the track seals are pressed tightly against the struts after adhesive application.
Run the unit for approximately one (1) hour after application to ensure proper alignment of track seals.
View tight positioning of the track seals against the strut.
Most adhesives require 24 hours for a complete curing process.
Page 17

W riti ng o n
TO P
W H I T E W IR E S
B L A C K W IRE S
FR O M
P O W E R S U PP LY
TO C IR C UIT
B O AR D
FA S T E N IN G
C L I P S
www.ActiveForever.com
ON / OFF SWITCH REPLACEMENT INSTRUCTIONS
TOOLS REQUIRED:
On / Off Switch — Reorder No. 1507
Pliers
Caution:
To avoid electric shock unplug the unit prior to beginning this procedure.
INSTRUCTIONS:
Turn the Power Switch off and unplug unit from the wall outlet.
Remove the Control Box Cover. (5 Phillips Screws or 4 Phillips Screws for newer models).
Unplug the two (2) white and two (2) black wires from the back of the On/Off Switch.
Use the pliers to squeeze the fastening clips and push the switch out.
Press the new On/Off Switch into position.
Plug in the white and black wires as indicated (see illustration).
Replace the Control Box Cover.
Page 18

www.ActiveForever.com
CIRCUIT BOARD REPLACEMENT INSTRUCTIONS
TOOLS REQUIRED:
Phillips Head Screwdriver
Small Non-magnetic Phillips Head Screwdriver
INSTRUCTIONS:
Turn the power off.
Remove the top cover of the Control Box (4 Phillips Head Screws).
Unplug the motor, power, pendant and knee potentiometer connectors.
Using the non-magnetic screwdriver, remove the 4 screws holding the Circuit Board in place.
Replace with the new board and secure with the 4 screws.
Plug in the motor, power, pendant and potentiometer connectors.
Follow the calibration procedure.
Replace the top cover.
Page 19

www.ActiveForever.com
CALIBRATION PROCEDURE MODEL 1800
TOOLS REQUIRED:
Straight Edge, Protractor, 3/32 Allen Wrench, Phillips Screwdriver, 2 Position Jumper, Voltmeter
INSTRUCTIONS:
Turn the machine off. Remove knee potentiometer cover. Remove top cover of the unit.
Remove 2 position jumper from J5.
Place the 2 position jumper on the zero (0) position pins 1 and 2 (Counting from the Foot Plate down)
Turn the machine on. Listen for 2 audible beeps.
Using the Start/Stop button on the Pendant move the unit into the zero position. Use a straight edge to determine the zero.
Stop the unit in that position.
Verify the Knee Pot Voltage is 2.0 volts DC.
To test this use a volt meter and touch the red lead to the red wire and the black lead to the black wire on the knee pot.
If reading is not at 2.0 volts DC then you must loosen the knee pot clamps and turn the knee pot either clockwise or counter
clockwise to achieve 2.0 volts (Warning while doing this procedure watch the tension on the 3 wires coming from the knee pot
cable) Then tighten clamps to lock the knee pot down when 2.0 volts is achieved
Remove the 2 position jumper from zero (0) position Pins 1 and 2. Listen for audible beeps.
Place 2 position jumper on the 90 degree position (90) Pins 3 and 4.
Using the Start/Stop button on the Pendant move the unit into the 90 degree position (90) using a protractor or similar device.
Stop the unit in that position.
Remove the 2 position jumper from the 90 degree position pins 3 and 4. Listen for audible beeps.
Verify the hand control settings are 0-Pause, Max- Speed, -5 extension and 110 flexion.
Press the start/stop button on the hand control and allow the unit to travel through the full range of motion.
If the unit travels through the full range of motion without binding then unit has been successfully calibrated.
Reinstall 2 position jumper on its original settings pins 5 and 6 or pins 6 and 7
2 position jumper on pins 5 and 6 (Pause in Extension only)
2 position jumper on pins 6 and 7 (Pause in Extension and Flexion)
Page 20

www.ActiveForever.com
CALIBRATION PROCEDURE MODEL 1850
TOOLS REQUIRED:
Straight Edge, Protractor, 3/32 Allen Wrench, Phillips Screwdriver
INSTRUCTIONS:
Turn power off
Remove knee potentiometer cover
Remove top cover from the unit
Move SW1 slide switch located at the bottom left of the board from run to calibrate
Using the up and down arrows move the unit to the zero position
Use a straight edge to determine zero
Look for these values on the hand control
Ext. window (Letter F with a red dot)
ROM Window (2) Flexion Window (490) reading this as 2490 (2490 represents the zero setting)
If these values are not present then the unit is out of calibration
If the above values do not appear on the hand control then it is necessary to move the knee pot to gain these values. To move
the knee pot, loosen the clamps on the knee pot and turn the knee pot clockwise or counterclockwise to achieve the above
readings. Once these values have been achieved then tighten the knee pot clamps.
To lock in the zero (0) position, depress the ext. button 1 time and listen for an audible beep.
By depressing the UP arrow button you can move the unit into the 90 degree (90) position, using a protractor or similar device,
stopping at this position
Once in the 90 degree position (90) and if you are in the approximate range a red dot will be visible in the flexion window.
If the red dot is visible then depress the flexion button 1 time and listen for an audible beep.
Switch unit from calibration mode to run mode
Set hand control at -5 extension, 110 Flexion, 0 pause, and 10 speed
Depress start/stop switch on the hand control and allow unit to travel through full range of motion. If the unit travels the full
range of motion without binding then the unit has been calibrated successfully.
Page 21

www.ActiveForever.com
CALIBRATION PROCEDURE MODEL DC2480
TOOLS REQUIRED:
Straight Edge, Protractor, 3/32 Allen Wrench, Phillips Screwdriver
INSTRUCTIONS:
Turn power o
Remove knee potentiometer cover
Remove top cover from the unit
Move SW1 slide switch located at the bottom left of the board from run to calibrate
Using the up and down arrows move the unit to the zero position
Use a straight edge to determine zero
Look for these values on the hand control
Ext. window (Letter F with a red dot)
ROM Window (2) Flexion Window (795) reading this as 2795 (2795 represents the zero setting)
If these values are not present
If the above values do not appear on the hand control then it is necessary to move the knee pot to gain these values. To move
the knee pot, loosen the clamps on the knee pot and turn the knee pot clockwise or counterclockwise to achieve the above
readings. Once these values have been achieved then tighten the knee pot clamps.
To lock in the zero (0) position, depress the ext. button 1 time and listen for an audible beep.
By depressing the UP arrow button you can move the unit into the 90 degree (90) position, using a protractor or similar device,
stopping at this position
Once in the 90 degree position (90) and if you are in the approximate range a red dot will be visible in the exion window.
If the red dot is visible then
Switch unit from calibration mode to run mode
Set hand control at –10 extension, 120 Flexion, 0 pause, and 10 speed
Depress start/stop switch on the hand control and allow unit to travel through full range of motion. If the unit travels the full
range of motion without binding then the unit has been calibrated successfully.
then the unit is out of calibration
depress the exion button 1 time and listen for an audible beep.
 Loading...
Loading...