ACON SARS-CoV-2 User manual
Swiss Point of Car
e
SARS-CoV-2 Antigen Rapid Test
Package Insert
A rapid test for the qualitative detection of SARS-CoV-2 nucleocapsid antigens in nasal swab specimens.
For professional in vitro diagnostic use only.
The SARS-CoV-2 Antigen Rapid Test is a lateral flow chromatographic immunoassay for the qualitative
detection the nucleocapsid protein antigen from SARS-CoV-2 in nasal swab specimens directly from
individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the
onset of symptoms. The SARS-CoV-2 Antigen Rapid Test does not differentiate between SARS-CoV and
SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid antigen. This antigen is generally detectable
in upper respiratory samples during the acute phase of infection. Positive results indicate the presence of
viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to
determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses.
The agent detected may not be the definite cause of disease.
Negative results, from patients with symptom beyond seven days, should be treated as presumptive and
confirmed with a molecular assay, if necessary, for patient management. Negative results do not rule out
SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management
decisions, including infection control decisions. Negative results should be considered in the context of a
patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-
19.
The SARS-CoV-2 Antigen Rapid Test is intended for use by trained clinical laboratory personnel and
individuals trained in point of care settings. SARS-CoV-2 Antigen Rapid Test is intended to be used as an aid
in the diagnosis of SARS-CoV-2 infection.
The novel coronaviruses belong to the β genus.1 COVID-19 is an acute respiratory infectious disease. People
are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of
infection; asymptomatic infected people can also be an infectious source. Based on the current
epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main
manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and
diarrhea are found in a few cases.
The SARS-CoV-2 Antigen Rapid Test is a qualitative membrane based chromatographic immunoassay for
the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in human nasal swab
specimens.
When specimens are processed and added to the test cassette, SARS-CoV-2 antigens, if present in the
specimen, will react with the anti-SARS-CoV-2 antibody-coated particles, which have been pre-coated on the
test strip.
The mixture then migrates upward on the membrane by capillary action. The antigen-conjugate
complexes migrate across the test strip to the reaction area and are captured by a line of antibody bound on
the membrane. Test results are interpreted visually at 15-30 minutes based on the presence or absence of
visually
colored lines.
To serve as a procedure control, a colored line will always appear in the control line region indicating that
proper volume of specimen has been added and membrane wicking has occurred.
The test cassette contains anti-SARS-CoV-2 antibodies. The positive control swab contains SARS-CoV-2
recombinant antigen pre-coated on the swab.
For professional in vitro diagnostic use only. Do not use after the expiration date.
Do not eat, drink, or smoke in the area where the specimens or kits are handled.
Do not use the test if the pouch is damaged.
Handle all specimens as if they contain infectious agents. Observe established precautions against
biological hazards throughout testing and follow the standard procedures for proper disposal of specimens.
Wear protective clothing such as laboratory coats, disposable gloves, mask and eye protection when
specimens are being tested.
The used test should be discarded according to local regulations. The used test should be considered
potentially infectious and be discarded according to local regulations.
Humidity and temperature can adversely affect results.
REF L031-11823A English
INTENDED USE
SUMMARY
PRINCIPLE
REAGENTS
PRECAUTIONS
This package insert must be read completely before performing the test. Failure to follow directions in insert
may yield inaccurate test results.
The test line for a high viral load sample may become visible within 15 minutes, or as soon as the sample
passes the test line region.
The test line for a low viral load sample may become visible within 30 minutes.
STORAGE AND STABILITY
The kit can be stored at temperatures between 2 - 30 °C.
The test is stable until the expiration date printed on the sealed pouch.
The test must remain in the sealed pouch until use.
DO NOT FREEZE.
Do not use after the expiration date.
MATERIALS
Materials Provided
Test Cassettes Extraction Tubes
Positive Control Swab Negative Control Swab
Disposable Swabs* Extraction Buffer
Package Insert
* The Disposable Swabs are produced by another manufacturer.
Materials Required But Not Provided
Personal Protective Equipment Timer
SPECIMEN COLLECTION AND PREPARATION
The SARS-CoV-2 Antigen Rapid Test can be performed using nasal swab specimens.
Testing should be performed immediately after specimen collection, or at most within one (1) hour after
specimen collection, if stored at room temperature (15-30°C).
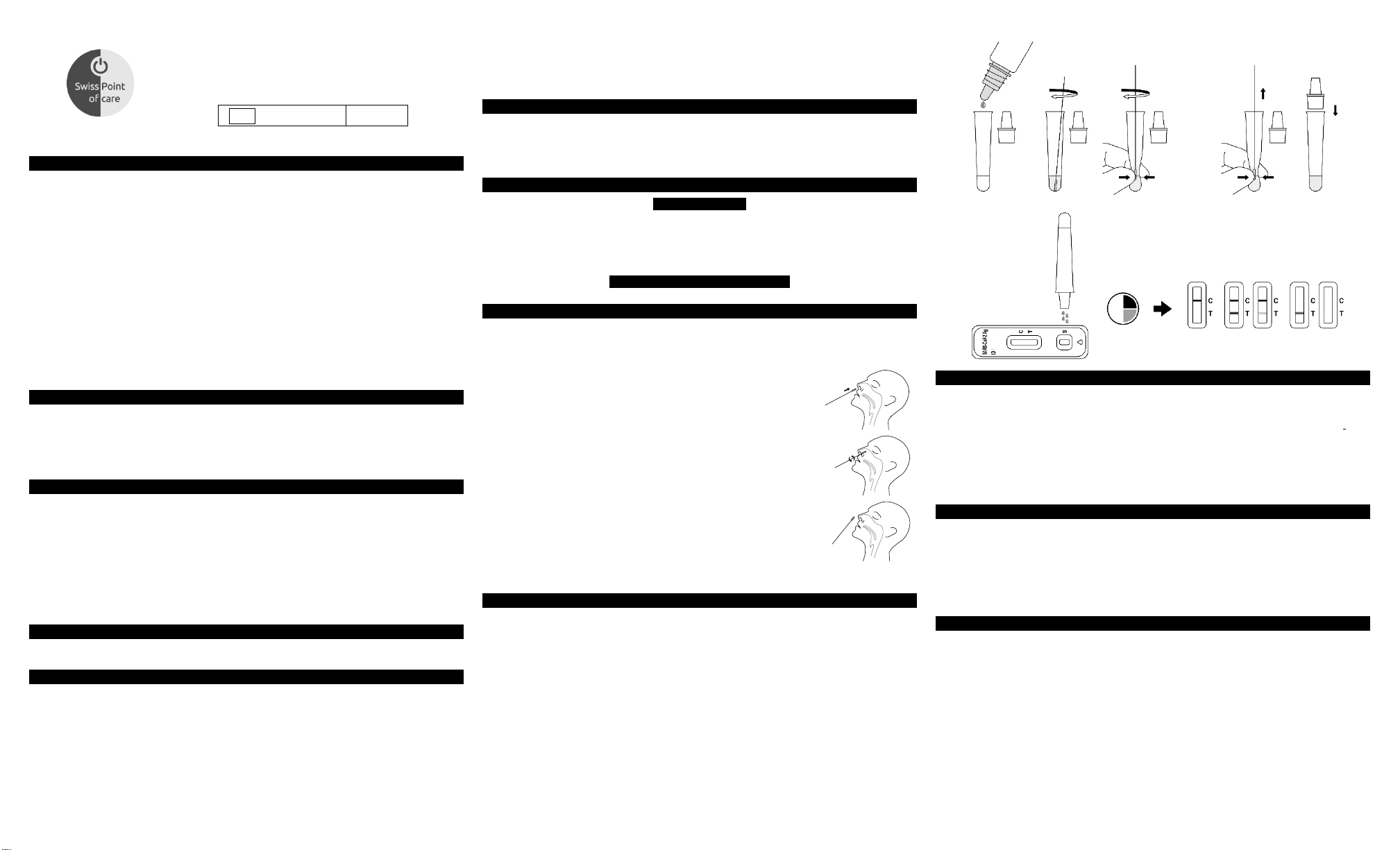
To collect a nasal swab sample:
1. Carefully insert a Disposable Swab, provided with your kit, into one
nostril. Using gentle rotation, push the swab up to 2.5 cm (1 inch) from
the edge of the nostril.
2. Rotate the swab 5 times against the mucosa inside the nostril to ensure
sufficient specimen collection.
3. Using the same swab, repeat this process in the other nostril to ensure
that an adequate amount of sample is collected from both nasal cavities.
4. Withdraw the swab from the nasal cavity. The specimen is now ready for preparation using the
extraction buffer tubes.
DIRECTIONS FOR USE
Allow the test and extraction buffer to reach room temperature (15-30 °C) prior to testing.
1. Use an extraction buffer tube for each specimen to be tested and label each tube appropriately.
2. Hold the extraction buffer bottle upside down vertically, then add approximately 300 μL (10~12 drops)
of extraction buffer to the extraction tube.
3. Insert the swab into the tube and swirl it for 30 seconds. Then rotate the swab at least 5 times while
squeezing the sides of the tube. Take care to avoid splashing contents out of the tube.
4. Remove the swab while squeezing the sides of the tube to extract the liquid from the swab.
5. Attach the dropper tip firmly onto the extraction buffer tube containing the sample. Mix thoroughly by
swirling or flicking the bottom of the tube.
6. Remove the test cassette from the foil pouch and use it as soon as possible.
7. Place the test cassette on a flat and clean surface.
8. Add the processed specimen to the sample well of the test cassette.
a. Invert the extraction buffer tube with the dropper tip pointing downwards and hold it vertically.
b. Gently squeeze the tube, dispensing 4 drops of the processed specimen into the sample well.
9. Wait for the colored line(s) to appear. The result should be read at 15-30 minutes. Do not read the
result after 30 minutes.
Rotate at least 5
times while
squeezing the tube
4 drops of the
processed
specimen
Swirl for 30
seconds
15-30 min.
Negative Positive Invalid
INTERPRETATION OF RESULTS
NEGATIVE: Only one colored control line appears in the control region (C).
in the test line region (T). This means that no SARS-CoV-2 antigen was detected.
POSITIVE:* Two distinct colored lines appear. One line in the control line region (C) and the other line in the
test line region (T). This means that the presence of SARS-CoV-2 antigen was detected.
*NOTE: The intensity of the color in the test line (T) may vary depending on the level of the SARS-CoV-2 antigen
present in the specimen. Therefore, any shade of color in the test line region (T) should be considered positive.
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect operation are the most
likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the
problem persists, discontinue using the test kit immediately and contact your local distributor.
(Please refer to the illustration above)
No apparent colored line appears
QUALITY CONTROL
Internal procedural controls are included in the test. A colored line appearing in the control line region (C) is
an internal procedural control. It confirms sufficient specimen volume and correct procedural technique.
Positive and Negative control swabs are supplied with each kit. These control swabs should be used to
ensure that the test cassette and that the test procedure is performed correctly. Follow the “DIRECTIONS
FOR USE” section to perform the control test.
The control swabs can be tested under any of the following circumstances:
1. When new lot of tests are used and/or when a new operator performs the test.
2. At periodic intervals as dictated by local requirements, and/or by the user’s Quality Control procedures.
LIMITATIONS
1. The SARS-CoV-2 Antigen Rapid Test is for in vitro diagnostic use only. The test should be used for the
detection of SARS-CoV-2 antigens in nasal swab specimens only. The intensity of the test line does not
necessarily correlate to SARS-CoV-2 viral titer in the specimen.
2. Specimens should be tested as quickly as possible after specimen collection and at most within the hour
following collection.
3. Use of viral transport media may result in decreased test sensitivity.
4. A false-negative test may result if the level of antigen in a sample is below the detection limit of the test
or if the sample was collected incorrectly.
5. Test results should be correlated with other clinical data available to the physician.
6. A positive test result does not rule out co-infections with other pathogens.
7. A positive test result does not differentiate between SARS-CoV and SARS-CoV-2.
8. A negative test result is not intended to rule out other viral or bacterial infections.
9. A negative result, from a patient with symptom onset beyond seven days, should be treated as
presumptive and confirmed with a molecular assay, if necessary, for clinical management.
(If the differentiation of specific SARS viruses and strains is needed, additional testing is required.)
PERFORMANCE CHARACTERISTICS
)
)
g
g
g
g
g
p
g
g
g
g
g
g
g
g
g
g
g
g
g
g
g
g
p
g
g
p
g
p
g
pyog
g
g
g
g
g
g
g
pray
y
y
pray
pray
pray
y
y
p
ACON
The performance of SARS-CoV-2 Antigen Rapid Test was established with 577 nasal swabs collected from
individual symptomatic patients who were suspected of COVID-19. The results show that the relative
sensitivity and the relative specificity are as follows:
SARS-CoV-2 Antigen
Rapid Test
Relative Sensitivity: 96.9% (92.8%-98.9%)* Relative Specificity: 99.5% (98.1%-99.9%)*
Accuracy: 98.8% (97.5%-99.5%)* *95% Confidence Intervals
Stratification of the positive samples post onset of symptoms between 0-3 days has a positive percent
agreement (PPA) of 98.7% (n=75) and 4-7 days has a PPA of 96.7% (n=60).
Positive samples with Ct value ≤33 has a higher positive percent agreement (PPA) of 98.7% (n=150) .
The LOD of SARS-CoV-2 Antigen Rapid Test was established using limiting dilutions of a viral sample
inactivated by gamma irradiation. The viral sample was spiked with negative human nasal sample pool into
a seral of concentrations. Each level was tested for 30 replicates. The results show that the LOD is 1.6*10
/mL.
TCID
50
Sample SARS-CoV-2 Concentration % Positive (Tests)
1.28*103 TCID50/mL 100% (30/30)
6.4*102 TCID50/mL 100% (30/30)
3.2*102 TCID50/mL 100% (30/30)
1.6*102 TCID50/mL 96.7% (29/30)
8*10 TCID50/mL 0% (0/30)
Clinical Sensitivity, Specificity and Accuracy
Clinical Performance for SARS-CoV-2 Antigen Rapid Test
Method RT-PCR
Results Negative Positive
Negative 413 5 418
Positive 2 157 159
Total Results 415 162 577
Limit of Detection (LOD)
Total
Results
Cross-Reactivity (Analytical Specificity) and Microbial Interference
Cross-reactivity was evaluated by testing a panel of related pathogens and microorganisms that are likely to
be present in the nasal cavity. Each organism and virus were tested in the absence or presence of heatinactivated SARS-CoV-2 virus at low positive level.
No cross-reactivity or interference was observed with the following microorganisms when tested at the
concentration presented in the table below. The SARS-CoV-2 Antigen Rapid Test does not differentiate
between SARS-CoV and SARS-CoV-2.
Potential Cross-Reactant Test Concentration
Adenovirus 1.14 x 106 TCID50/mL
Enterovirus 9.50 x 105 TCID50/mL
Human coronavirus
229E
Human coronavirus
OC43
Human coronavirus
NL63
Human
neumovirus
Meta
MERS-coronavirus 7.90 x 105 TCID50/mL
Influenza A 1.04 x 105 TCID50/mL
Virus
Influenza B 1.04 x 105 TCID50/mL
Parainfluenza virus 1 1.25 x 105 TCID50/mL
Parainfluenza virus 2 3.78 x 105 TCID50/mL
Parainfluenza virus 3 1.0 x 105 TCID50/mL
Parainfluenza virus 4 2.88 x 106 TCID50/mL
Respiratory syncytial
virus
Rhinovirus 3.15 x 105 TCID50/mL
Human coronavirus-
HKU1
1.04 x 10
2.63 x 10
1.0 x 10
1.25 x 10
3.15 x 10
1 x 10
5
TCID50/mL
5
TCID50/mL
5
TCID50/mL
5
TCID50/mL
5
TCID50/mL
5
copies/mL
Cross-Reactivity
(in the absence of
SARS-CoV-2 virus
No
3/3 ne
ative
No
3/3 ne
ative
No
3/3 ne
ative
No
3/3 ne
ative
No
3/3 ne
ative
No
ative
3/3 ne
No
3/3 ne
ative
No
3/3 ne
ative
No
ative
3/3 ne
No
3/3 ne
ative
No
3/3 ne
ative
No
ative
3/3 ne
No
3/3 ne
ative
No
3/3 ne
ative
No
ative
3/3 ne
No
3/3 ne
ative
Interference
(in the presence of
SARS-CoV-2 virus
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
No
No
No
No
No
No
No
No
No
No
No
No
No
No
No
No
Bordetella pertussis 2.83 x 109 CFU/mL
Chlamydia trachomatis 3.13 x 108 CFU/mL
Haemophilus influenza 1.36 x 108 CFU/mL
Legionella pneumophila 4.08 x 109 CFU/mL
Mycobacterium
tuberculosis
Mycoplasma
neumoniae
Staphylococcus aureus 1.38 x 107 CFU/mL
Bacteria
Staphylococcus
e
idermidis
Streptococcus
neumoniae
Streptococcus
Pneumocystis jirovecii-
2
Chlamydia pneumoniae 1×106 IFU/ml
Yeast Candida albicans 1.57 x 108 CFU/mL
The following substances, naturally present in respiratory specimens or that may be artificially introduced into
the nasal cavity or nasopharynx, were evaluated. Each substance was tested in the absence or presence of
SARS-CoV-2 virus at low positive level. The final concentration of the substances tested are listed below and
were found not to affect test performance.
Interfering
Substance
Endogenous
Afrin Original Nasal
S
ALKALOL Allerg
Relief Nasal Spra
Chloraseptic Max
Sore Throat
Lozenges
CVS Health
Fluticasone
Propionate Nasal
Spray
Equate Fast-Acting
Nasal S
Equate Sore Throat
Phenol Oral
Anesthetic S
Original Extra
Strong Menthol
Cough Lozenges
NasalCrom Nasal
S
NeilMed NasoGel
for Dr
Throat Lozenge
Zicam Cold Remedy
Antibiotic Mupirocin 10 mg/mL 3/3 negative 3/3 positive
enes
S. cerevisiae
Pseudomonas
aeru
inosa
Pooled human nasal wash
Active Ingredient Concentration
Biotin 2.4 mg/mL 3/3 negative 3/3 positive
Mucin 0.5% w/v 3/3 negative 3/3 positive
Whole Blood 4% v/v 3/3 negative 3/3 positive
Oxymetazoline 15% v/v 3/3 negative 3/3 positive
Homeopathic 1:10 Dilution 3/3 negative 3/3 positive
Menthol,
Benzocaine
Fluticasone
propionate
Phenylephrine 15% v/v 3/3 negative 3/3 positive
Phenol 15% v/v 3/3 negative 3/3 positive
Menthol 1.5 mg/mL 3/3 negative 3/3 positive
Cromolyn 15% v/v 3/3 negative 3/3 positive
Noses
Sodium
Hyaluronate
Dyclonine
drochloride
H
Galphimia glauca,
Luffa operculata,
Sabadilla
7
1.72 x 10
CFU/mL
7
CFU/mL
7.90 x 10
9
CFU/mL
2.32 x 10
8
CFU/mL
1.04 x 10
6
4.10 x 10
CFU/mL
7
8.63 x 10
CFU/mL
8
CFU/mL
1.87 x 10
Interfering Substances
1.5 mg/mL 3/3 negative 3/3 positive
5% v/v 3/3 negative 3/3 positive
5% v/v 3/3 negative 3/3 positive
1.5mg/mL 3/3 negative 3/3 positive
5% v/v 3/3 negative 3/3 positive
No
3/3 ne
ative
No
3/3 ne
ative
No
3/3 ne
ative
No
ative
3/3 ne
No
3/3 ne
ative
No
3/3 ne
ative
No
ative
3/3 ne
No
3/3 ne
ative
No
3/3 ne
ative
No
ative
3/3 ne
No
3/3 ne
ative
No
3/3 ne
ative
No
ative
3/3 ne
No
3/3 ne
ative
No
3/3 ne
ative
Results
(in the absence of
SARS-CoV-2 virus)
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
3/3 positive
(in the presence of
SARS-CoV-2 virus)
No
No
No
No
No
No
No
No
No
No
No
No
No
No
No
Results
Tam i fl u
Antibiotic Tobramycin 4 µg/mL 3/3 negative 3/3 positive
Mometasone
Furoate Nasal
Spray
Physiological
Seawater Nasal
Cleaner
Oseltamivir
Phos
hate
Mometasone
Furoate
NaCl 15%v/v 3/3 negative 3/3 positive
5 mg/mL 3/3 negative 3/3 positive
5%v/v 3/3 negative 3/3 positive
PRECISION
Within-run precision was determined using 10 replicates of specimens: negative control and SARS-CoV-2
antigen positive controls. The specimens were correctly identified >99% of the time.
Between-run precision was determined using 10 independent assays on the same specimen: negative
specimen and SARS-CoV-2 an tigen positive specimen. Three different lots of the SARS-CoV-2 Antigen Rapid
Test were tested using these specimens. The specimens were correctly identified >99% of the time.
Intra-Assay
Inter-Assay
BIBLIOGRAPHY
1. Shuo Su, Gary Wong, Weifeng Shi, et al. Epidemiology, Genetic recombination, and pathogenesis of
coronaviruses. Trends in Microbiology, June 2016, vol. 24, No. 6: 490-502
2. Susan R. Weiss, Julian L. Leibowitz, Coronavirus Pathogenesis, Advances in Virus Research, Volume
81: 85-164
Manufacturer
In vitro diagnostic
IVD
medical device
Consult instructions for
use
Index of Contents
Authorized representative in the European Community
SARS-CoV-2 Antigen
Negative Control Swab
Positive Control Swab
Extraction Tubes
Extraction Buffer
Disposable Swabs
SARS-CoV-2 Antigen Rapid Test
Index of Symbols
LOT
Contains sufficient for
<n> tests
Use-by date
Batch code
REF
SARS-CoV-2 Antigen
Negative Control Swab
Positive Control Swab
Extraction Tubes
Extraction Buffer
Disposable Swabs
SARS-CoV-2 Antigen Rapid Test
Temperature limit
Do not reuse
Catalogue number
Date of manufacture
Biotech (Hangzhou) Co., Ltd.
No.210 Zhenzhong Road, West Lake
District, Hangzhou, P.R.China, 310030
Distributor:
MedNet GmbH
Borkstrasse 10
48163 Muenster, Germany
www.swisspointofcare.com
Number: 1151255102
Effective date: 2020-xx-xx
 Loading...
Loading...