REF NTSC/PAL/VGA: ICN-0564, ICN-0565
Invisio™ ICN Flexible CystoNephroscope
Operations and Technical Manual
ACMI Corporation
136 Turnpike Road • Southborough, MA 01772-2104 • USA
(888) 524-7266 or (262) 639-7205 • Fax: (262) 681-4038 • www.acmicorp.com
Authorized European Representative: MDCI, Ltd.
Arundel House • 1 Liverpool Gardens • Worthing, West Sussex BN11 1SL • United Kingdom
™ Trademark of ACMI Corporation or its Subsidiaries in the U.S. and/or other countries of the world Printed in USA
© 2004 ACMI Corporation, All Rights Reserved U.S. Patent Nos. 5,938,588; 6,171,235; 6,475,140; 6,485,411; other patents pending
99-1089 REV 10 1104

Table of
Contents
Repair/Return/Warranty ...................................................................... 3
Warnings and Cautions ....................................................................... 4
Section 1.0 Unpacking and Initial Inspection .......................................... 5
Section 2.0 General Description and Intended Use ................................... 5
Section 3.0 Description of Components................................................. 6
Section 3.1
Section 3.2 Technical Specifications ....................................................... 6
Section 4.0 Operation ...................................................................... 7
Section 4.1
Section 4.2 Optical Field Inspection ........................................................ 7
Section 4.3 Deflection Controls ............................................................... 8
Section 4.4 Leakage Tester ...................................................................... 8
Section 4.5 A
Section 4.6 Rotating Working Channel/Irrigation Port............................. 9
Section 4.7 Strain Relief .......................................................................... 9
Section 4.8 Cable Compensation ............................................................. 9
Section 5.0 Procedure .................................................................... 10
Section 5.1 Working Channel ................................................................ 10
Section 5.2 White Balancing .................................................................. 10
Section 5.3 Focusing ............................................................................. 10
Section 5.4 Remote Head Button Setup ................................................ 10
Section 5.5 On-Screen-Display (OSD) System ...................................... 11
Section 5.6 OSD Options and Navigation .............................................. 11
Section 5.7 Inspection ........................................................................... 12
Section 6.0 Troubleshooting Guide ..................................................... 12
Section 6.1 Troubleshooting: Invisio ICN Flexible CystoNephroscope... 12
Section 6.2 Troubleshooting: Video Image ............................................ 13
Section 7.0 General Care................................................................. 14
Section 8.0 Maintenance ................................................................. 14
Section 8.1 Cleaning - Invisio ICN Flexible CystoNephroscope ............. 15
Section 8.2 Cleaning the Optics on the Invisio ICN Flexible
Section 8.3 Cleaning the Invisio ICN Connector and Receptacle ........... 15
Section 8.4 Cleaning the Invisio IDC Digital Controller .......................... 15
Section 8.5
Section 8.6 Autoclave Sterilization ........................................................ 15
Section 8.7 Ethylene Oxide Sterilization ................................................ 16
Section 8.8 STERIS SYSTEM 1™ Sterilization ........................................ 16
Section 8.9 STERRAD® Sterilization ...................................................... 16
Section 9.0 Storage ....................................................................... 16
Section 10.0 Theory of Operation ........................................................ 16
Section 10.1 General ............................................................................... 16
Invisio ICN Flexible CystoNephroscope & Accessories ..............
Inspection of Invisio ICN Flexible CystoNephroscope ..............
UTOSEAL Feature ................................................................... 9
CystoNephroscope ............................................................. 15
Disinfection - Invisio ICN Flexible CystoNephroscope .............
15
6
7
2
99-1089 REV 10

Repair/Return/
Warranty
Repair/Return
All returns for credit must have prior written authorization. All medical devices returned to ACMI for any
reason must be 1) disassembled, 2) cleaned, and 3) high-level disinfected or sterilized in accordance with the
product’s instructions for use or labeling, and shipped in accordance with ACMI’s return procedures (available
upon request) and all applicable regulations. Prior to making any return, call ACMI Customer Service at (888)
524-7266.
Return to (worldwide):
ACMI Corporation – Stamford
Attn: Product Repair Dept.
300 Stillwater Avenue
Stamford, CT 06902-3695. USA
Ph: (888) 524-7266 or (262) 639-7205
Fax: (262) 681-4038
Limited Express Warranty
SHOULD THE PRODUCT BECOME INOPERABLE, DURING NORMAL AND PROPER USE IN ACCORDANCE
WITH APPLICABLE INSTRUCTIONS, WITHIN THE TIME FRAME SPECIFIED BELOW FROM THE DATE OF SHIPMENT, ACMI WILL REPAIR OR REPLACE THE PRODUCT, AT ITS SOLE OPTION, AT NO CHARGE. ACMI MAKES
NO OTHER WARRANTIES WITH RESPECT TO THE PRODUCTS AND EXPRESSLY DISCLAIMS ALL OTHER
WARRANTIES, EXPRESS OR IMPLIED, AS TO MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE
OR ANY OTHER MATTER. IN NO EVENT SHALL ACMI BE LIABLE FOR ANY CONSEQUENTIAL DAMAGES.
This Warranty runs only to the original user and may be voided if the product(s) are serviced or repaired by
anyone other than ACMI or an organization duly authorized by ACMI for such purpose.
Invisio ICN Flexible CystoNephroscope (excluding cable).................................................................. 2 years
Invisio ICN Flexible CystoNephroscope cable only ............................................................................... 1 year
Accessories ........................................................................................................................................... 90 days
SP-1102
WA-1201
99-1089 REV 10
3

Warnings and
Cautions
The following warnings and cautions apply to the use, care, and/or maintenance of the ACMI® Invisio ICN
Flexible CystoNephroscope System. Failure to comply with, or abide by, any warning or caution set forth in
this manual will void the video system’s Limited Express Warranty.
Warnings
(indicate that a danger to life or health can result from misusing the equipment)
1. SERVICE WARNING AND DISCLAIMER NOTICE: It is recommended that the medical equipment for
which these documents apply be returned to ACMI for servicing or repair. Service or repair
provided by any party other than ACMI or an ACMI-authorized repair facility may result in the user
and/or repair facility being liable and responsible for damages, including patient or user injuries,
arising from such servicing or repair, and any such servicing or repair shall void the
manufacturer’s Limited Express Warranty, if any, applicable to such medical equipment.
2. To prevent electric shock and breakage of seals, do not remove covers on the Invisio ICN Flexible
CystoNephroscope or Invisio IDC Digital Controller as a potential electric shock hazard exists. Refer
all repairs to ACMI-authorized service personnel.
3. Study this manual and other labeling thoroughly for safe handling and storage. Misuse of the
equipment can cause injury to the patient and could have an adverse effect on the procedure being
performed. Do not drop equipment, or allow it to be struck by other objects.
4. Procedures should be performed only by persons with adequate training and preparation. Consult the
medical literature regarding techniques, complications, and hazards prior to any procedure.
5. Do not use electromedical energy sources in the presence of flammable detergents, anesthetics,
nitrous oxide (N2O), or oxygen.
6. Consult the operating manuals of all electromedical energy sources used with endoscopic instruments
for appropriate instructions, warnings, and cautions prior to use. Such sources of energy include
electrical, electrohydraulic, electrosurgical, heat, hydraulic, laser, light, pressure, sound, ultrasound,
and vacuum.
7. Make no repairs to any Invisio ICN Flexible CystoNephroscope or Invisio IDC Digital Controller
Personal injury or damage to units may result. Refer units to ACMI-authorized service personnel for
repair.
8. Never look directly into the light emitted from the Invisio ICN Flexible CystoNephroscope. Damage to
the eyes can result.
9. Keep the distal tip of any electrode, probe, laser fiber, or other ancillary device in the field of view at all
times when active.
10. Use only those lubricants specified in the labeling.
11. Prior to use, examine each electrode and its insulation for damage; do not use if damaged.
12. Follow the disinfectant or sterilizer manufacturer’s recommended procedures and cautions.
13. Do not use disinfectant solutions that contain long-life surfactants; such solutions can leave
conductive residues.
14. Do not use an instrument that fails to meet the criteria stated in the labeling or that has been
damaged.
15. When instruments from different manufacturers are used together, verify that any isolation or
grounding is not violated.
16. Follow the labeling instructions regarding the disposal or reuse of accessories. Reuse of disposable
accessories could compromise patient safety.
.
® Registered trademark of ACMI Corporation or its Subsidiaries in the U.S. and /or other countries of the world
4
99-1089 REV 10

Warnings and Cautions
(continued)
Cautions
(indicate that equipment or other property may be damaged or may malfunction by misuse)
1. Pay close attention to the care, cleaning, disinfection, and sterilization instructions in this manual. Any
deviation can cause damage and void ACMI’s Limited Express Warranty. Do not steam sterilize the
Invisio ICN Flexible CystoNephroscope or Invisio IDC Digital Controller.
2. To ensure continued satisfactory performance, perform the prescribed inspections and operational
tests as recommended.
3. Thoroughly check all electrical cables and plugs before each use and replace any which are damaged
or excessively worn. Do not use if damage is suspected or discovered.
4. Check the outer surface of the flexible shaft prior to each use to make certain that the instrument is
free of any cuts, holes, rough surfaces, sharp edges, or protrusions. Do not use if damage is
suspected or discovered.
5. Do not insert a wet connector into the Invisio IDC Digital Controller receptacle, as poor video
performance and/or damage to the system may result.
6. These instruments contain no user-serviceable parts. Refer all repairs to ACMI-authorized service
personnel.
7. Connect electromedical energy source power cables only to properly wired grounding receptacles.
8. The Invisio ICN Flexible CystoNephroscope is listed by CSA as meeting Medical and Dental Equipment
Standard UL 2601 and CSA Standard 22.2 No. 601 and IEC-601.
9. Prior to use of a cardiac defibrillator, remove endoscopic instruments from the patient. Failure to
remove an endoscopic instrument from a patient during use of a cardiac defibrillator could result in
damage to the instrument due to the discharge of the cardiac defibrillator.
10. Testing to ensure that chassis leakage does not exceed the allowable levels per the appropriate
standard should be performed at least once each year. ACMI will make available on request circuit
diagrams, component part lists, descriptions, calibration instructions, or other information which will
assist the user’s appropriately qualified technical personnel to repair those parts of equipment which
are designated by ACMI as repairable.
11. Do not adjust electronic circuitry. Electronic circuitry is set at the factory and no further adjustment
is necessary.
12. Never remove the Invisio ICN Flexible CystoNephroscope from the Invisio IDC Digital Controller by
pulling on the cable. Always pull the insertion (connector) body to remove the cable from the IDC
Digital Controller.
13. U.S. Federal law restricts this device to sale by or on the order of a physician.
99-1089 REV 10
Section 1.0
Unpacking and Initial Inspection
Upon receipt, examine the shipping carton and its contents for signs of damage. Check for rattling or loose
material inside. Examine the Invisio ICN Flexible CystoNephroscope, Invisio IDC Digital Controller, and
accessories for damage. Do not use a damaged product—contact ACMI Customer Service at (888) 5247266 or (262) 639-7205.
It is very important that upon unpacking the equipment the proper video format selection is made: PAL,
VGA (Progressive Scan), or NTSC. See Invisio IDC Digital Controller Operations and Technical Manual
80819 for more detailed instructions.
Section 2.0
General Description and Intended Use
The Invisio ICN Flexible CystoNephroscope System consists of a Digital Flexible CystoNephroscope with an
integrated cable, accessories, an Invisio IDC Digital Controller, and all required video connection cables.
The ACMI Invisio ICN Flexible CystoNephroscope System (which includes the Invisio ICN Flexible
CystoNephroscope and Invisio IDC Digital Controller) is intended for use to examine body cavities, hollow
organs and canals in the body, in the urinary tract, and can be used percutaneously to examine the interior
of the kidney; and using additional accessories, can be used to perform various diagnostic and therapeutic
procedures.
5

Section 3.0
Description of Components
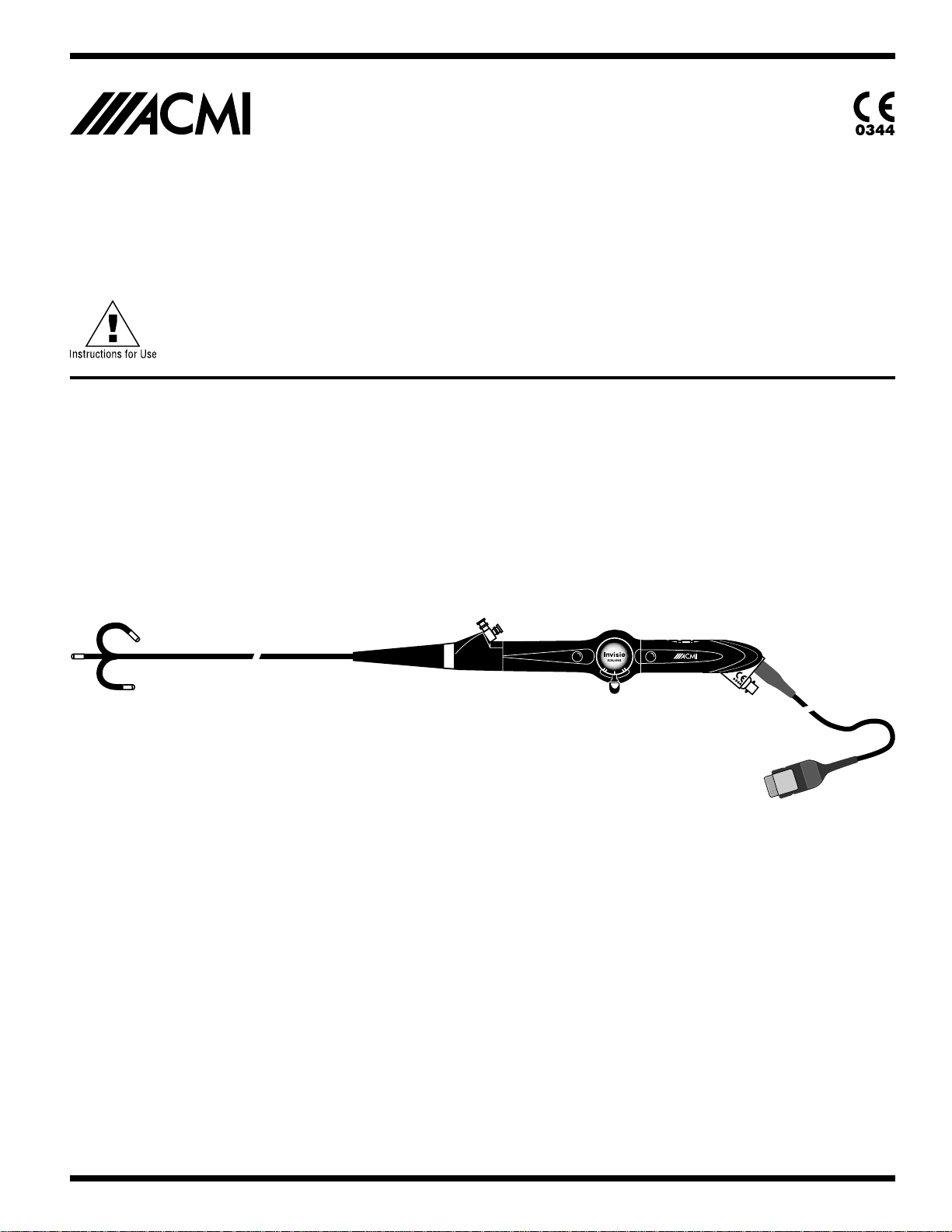
Invisio ICN Flexible CystoNephroscope
The distal tip of the ICN Flexible CystoNephroscope contains a digital imaging sensor that converts light
energy into an electrical signal for the production of an intra-operative video image that is displayed on a
monitor. The three remote push buttons on the CystoNephroscope allow fingertip control of ICN System
functions, as well as control of external devices and accessories.
4
3
7
8
2
6
1
5
10
6
9
Figure Identifications
1. Deflection Control Lever: UP and DOWN
2. Programmable Remote Control Buttons
3. Working Channel
4. Irrigation Port
6
5. Leak Test Valve
6. Strain Reliefs
7. Flexible Shaft
8. Deflection Section
9. Insertion (Connector)
10. Card Edge Printed Circuit Board Plug
Section 3.1 Invisio ICN Flexible CystoNephroscope & Accessories
Invisio ICN Flexible CystoNephroscopes
ICN-0564 .......... Invisio Flexible CystoNephroscope, 5 mm, 37 cm, 6.4 Fr channel
ICN-0565 .......... Invisio Flexible CystoNephroscope, backward articulating, 5 mm, 37 cm, 6.4 Fr channel
ICN-0564 and ICN-0565 are compatible with DCN-1500 and IDC-1000 controllers with software version
3.1 or higher. For controller software upgrades, contact ACMI Customer Service or your local ACMI
representative.
Accessories
Remote Printer Cable, Sony Printers .............................. MV-10350
Remote Printer Cable, Toshiba Printers ......................... MV-10351
BNC Cables, 4 ft. (2/pkg) ................................................ MV-14024
BNC Cables, 12 ft. (2/pkg) .............................................. MV-9144
BNC Cables, 40 ft. (2/pkg) .............................................. MV-9144-40
Y/C (S-Video) Cables, 4 ft. (2/pkg) ................................. 103017-1
Y/C (S-Video) Cables, 12 ft. (2/pkg) ............................... MV-9162
Y/C (S-Video) Cables, 30 ft. (2/pkg) ............................... MV-9162-30
RGB (Component) Cable, 6 ft. ........................................ IDC-0110
VGA (Progressive Scan) Cable, 6 ft. ............................... IDC-0100
Power Cables, 4 ft., 120 V (2/pkg) ................................. MV-10428
Connector Maintenance Kit, small…. ............................. MV-10454
Connector Maintenance Kit, large… ............................... MV-10455
Section 3.2 Technical Specifications
(for reference only—typical measurements/performance)
Invisio ICN-0564 and ICN-0565 Flexible CystoNephroscopes (typical)
Working Length: 37 cm
Distal Tip: 4.87 mm (14.6 Fr)
Shaft: 5.0 mm (15 Fr)
Working Channel: 2.1 mm (6.4 Fr)
Active Deflection: 210° up
Leak Test Valve: Yes
AUTOSEAL
(no vent valve for sterilization): Yes
Minimum Bend Radius: 0.30 inches (7.6 mm)
Field of View: 110° in air
Focus: Automatic 5–50 mm
Direction of View: 0°
160° down
™
6
99-1089 REV 10

Section 3.0
(continued)
Section 3.2.1. Safety Specifications
This equipment meets the Safety Requirements of–
Type Construction: Portable Equipment
Protection Against Electric Shock Hazards–
Class of Protection: Class 1
Degree of Protection: Type BF
Protection Against the Ingress of Water: Ordinary
Rated Operation: Continuous Operation
Other Safety Information:
Equipment is not for use in presence of flammable anesthetics (gases) or detergents.
Standard Production Line Safety Tests:
a) Dielectric withstanding test conditions—Specified Vms for 1 minute. No breakdown.
b) Leakage current test conditions—To the requirements listed in IEC-601-1 Table IV for Type BF equipment.
NOTE: Use of electrically active surgical devices in the working channel may increase the patient
leakage currents.
IEC 601-1: 1988 IEC/ CEI
IEC 601-1 AMEND. 1 1991-11
IEC 601-1 AMEND. 2 1995-03
IEC 601-1-2: 1993-04 IEC/ CEI
IEC 601-2-18: 1986-08 IEC/ CEI
UL 2601
CSA 22.2 NO. 601-1
EN 60601-1: 1990
EN 60601-1 AMEND.A1 1993
EN 60601-1 AMEND.A2 1995
EN 60601-1-2: 1993
Section 4.0
Operation
It is important that the user perform an operational and functional test of the instrument(s) prior to each
use. If an irregularity or substandard function is suspected, the instrument(s) should not be used. Notify
ACMI Customer Service immediately.
Section 4.1 Inspection of Invisio ICN Flexible CystoNephroscope
Perform the following steps before using the instrument—do not use if damage is found.
1. Visually inspect, and feel with fingertips, the entire surface of the shaft (including the distal tip) for
dents, protrusions, holes, or other irregularities. Examine the instrument in all deflection modes: nondeflected (straight), fully deflected up, and fully deflected down.
CAUTION: Never force the flexible tip into a straight or flexed position while holding the deflection
control—this can damage the control mechanisms.
2. Ensure that irrigant flows freely and that accessories pass easily through the Invisio ICN Flexible
CystoNephroscope working channel.
Section 4.2 Optical Field Inspection
Clean all lens surfaces, including the optical window, with a gauze pad or Q-tip moistened with 70%
isopropyl alcohol. Do not use if image on the monitor remains distorted or cloudy after cleaning.
While supporting the distal tip, position the Invisio ICN Flexible CystoNephroscope on an object 12 mm
away. The image on the monitor should be crisp and clear.
Refer to ACMI document
information.
Quality Check of Flexible and Semi-Rigid Endoscopes
(PI136) for additional
99-1089 REV 10
7

Section 4.0
(continued)
Section 4.3 Deflection Controls
Hold the Invisio ICN Flexible CystoNephroscope with the shaft extended downward with the irrigation port
pointing upward; place the thumb on the deflection control lever. When the deflection lever is in the middle
(or neutral) position (1) the tip will be straight. Logical deflection moves the tip up 210° when the lever is
1
2
2
3
1
3
moved up or pushed back (2) and down 160° when the lever is moved down or pushed forward (3).
CAUTION: Never bend the deflection sections of the shaft by the fingers as this can damage the
deflecting mechanism.
NOTE: The ICN-0565’s deflection control is international-style: pushing the deflection lever forward
articulates the distal tip “up” and pushing the lever back articulates the distal tip “down” (opposite to the
logical ICN-0564 deflection).
Section 4.4 Leakage Tester
The ACMI Invisio Leakage Tester enables the surgical staff to easily verify the integrity of the flexible
endoscope’s working channel.
Note: The leakage tester connector and air vent must both be completely dry.
1. Secure the leakage tester connector fitting (1) to the air vent (2) located on the proximal end of the
endoscope. Rotate the leakage tester connector so as to lock in place. Proper connection will require
300
20 280
40 260
80 220
60 240
3
1
100 200
120 180
100 200
alignment of the leakage tester connector with the pins located on the air vent and clockwise rotation
of the leakage tester connector.
2. Pressurize the scope by pumping the hand bulb (3) until the indicator on the gauge is in the range of
140–200 mmHg. DO NOT overpressurize as this may cause serious damage to the scope.
3. Monitor the gauge on the leakage tester to determine if the indicator remains in the range of 140–200
mmHg. If the indicator drops from this region rapidly, then a major leak has been detected.
2
4. Release the pressure release valve (4) on the leakage tester. After the gauge indicates ‘0’ (or below 40
mmHg), disconnect the leakage tester from the scope.
If a leak is detected, disconnect the leakage tester connector and verify that it and the air vent are
completely dry. Reattach and repeat steps 1, 2, and 3 above to confirm whether a leak is present. If
confirmed, DO NOT use endoscope. Contact ACMI customer service immediately.
4
8
99-1089 REV 10

Section 4.0
(continued)
Section 4.5 AUTOSEAL
The Invisio ICN Flexible CystoNephroscope is equipped with AUTOSEAL™ technology. The AUTOSEAL feature
eliminates the need to attach a venting valve, which allows for ventilation of ethylene oxide gas (EO) from
the instrument after sterilization.
Section 4.6 Rotating Working Channel/Irrigation Port
The working channel and irrigation port each rotate ±45°; both have Luer lock fittings.
An adjustable working channel seal (
detached by turning counterclockwise and pulling up. The ABP will accept working instruments up to 6 Fr in
diameter.
A standard, floppy tip .038" guidewire may be used to check the integrity of the channel. If an accessory
does not pass easily even though the guidewire passes freely, the accessory may be too large. If the
guidewire does not pass freely, contact ACMI Customer Service.
Section 4.7 Strain Relief
The external strain relief covers the proximal portion of the shaft. The strain relief protects the shaft during
use and storage. Do not bend the shaft sharply as light fiber breakage and/or shaft damage can result.
™
Feature
REF ABP) can be attached by turning clockwise and pushing down, and
Section 4.8 Cable Compensation
The Invisio ICN Flexible CystoNephroscope is equipped with a patented cable compensating mechanism to
guard the pull cables against excessive loads.
99-1089 REV 10
™ Trademark of ACMI Corporation or its Subsidiaries in the U.S. and/or other countries of the world
9

Section 5.0
Procedure
1. Attach the Irri-Flo™ Tubing (REF 5920000) (or similar tubing) to the irrigation port. Attach a 30 cc
sterile syringe to the three-way stopcock to provide controlled irrigation. Attach a self-sealing biopsy
seal to the accessory port (see Section 4.6 Rotating Working Channel/Irrigation Port) for insertion of
appropriate-size accessories.
2. When transporting the instrument, support the tip of the shaft with one hand and the proximal
housing with the other to prevent damage to the shaft.
3. Accessories cannot be inserted when the tip is deflected; attempting to do so may perforate the
working channel.
4. When the endoscope is straight, less resistance is noted. When the tip is deflected, resistance
increases as the angle of deflection increases. Attempts to forcibly insert or withdraw accessory
devices can damage the instrument channel or accessory.
1
2
5. If the scope is deflected, it may be necessary to withdraw it slightly to straighten the tip, advance the
accessory to the distal tip, reposition the scope, and then slowly advance the accessory.
IMPORTANT: To introduce an accessory (1) into the working channel with seal (2), grasp the accessory
shaft close to the working channel entry and advance slowly, using repeated short strokes. If resistance
is encountered, slowly withdraw the accessory several millimeters, then continue introduction. DO NOT
FORCE ACCESSORY—see Section 6.1 for more information.
CAUTION: Before attempting to pass instruments or devices through the working channel of a flexible
endoscope, the entire working channel must be as straight as possible, with no tip deflection.
The zero degree direction of view will allow the device to enter the field of view close to the tip. It is
recommended that the device then be pulled just out of the field of view before further endoscope manipulation. This will assure easy advancement of the device once the endoscope has arrived at the pathology.
Attempting to pass instruments or devices through a deflected flexible endoscope will cause damage to
the working channel. This damage can result in leakage, requiring repair of your flexible endoscope.
ACMI’s Limited Express Warranty does not cover such damage.
ACMI recommends a 270 micron laser fiber for maximum deflection and access to the kidney. The
superior deflection capability of ACMI flexible endoscopes can exceed the minimum bend radius of
larger fibers. A larger fiber may break and be accidentally fired inside the working channel, damaging
the endoscope.
CAUTION: Sharp accessories, such as laser fibers and EHL probes, can cause damage to the working
channel if passed when the scope is deflected—pass these instruments only when the scope is not
deflected (straight).
NOTE: As irrigation flow diminishes with an accessory in the channel, pressurized irrigation may be
necessary.
WARNING: Before removing the endoscope from the patient, be sure the shaft is straight (not deflected).
Patient injury and possible damage to the instrument could result.
Immediately flush the working channel with sterile distilled water using a syringe to remove any blood or mucus.
Section 5.1 Working Channel
IMPORTANT: When the endoscope is straight, less resistance is noted. When the tip is deflected,
resistance increases as the angle of deflection increases. Attempts to force entry or withdrawal of
accessory devices—especially sharp devices such as laser fibers and electrohydraulic lithotripsy (EHL)
probes—can cause damage to the working channel or accessory.
Pass all accessories all the way through the endoscope before deflecting the tip whenever possible.
Section 5.2 White Balancing
The Invisio ICN System does not require white balancing as it is set at the factory.
Section 5.3 Focusing
The Invisio ICN Flexible CystoNephroscope requires no focusing.
10
Section 5.4 Remote Head Button Setup
1
2
3
The Invisio ICN Flexible CystoNephroscope is equipped with three remote head buttons on the top of the
hand piece to control ICN System features and peripheral devices. The middle button (2) is dedicated to
brightness, while the distal and proximal buttons (1, 3) are programmable through the On-Screen-Display
(OSD) system (see Sections 5.5 and 5.6).
™ Trademark of ACMI Corporation or its Subsidiaries in the U.S. and/or other countries of the world
99-1089 REV 10

Button Setup : Accessory 1
Button Setup : Accessory 2
Display Language : English
Display Logo : On
Exit
Button For Select Option
Button For Review
Section 5.0
(continued)
Section 5.5 On-Screen-Display (OSD) System
The OSD enables the configuration of system functions, as well as the customization of the remote button
functions to a specific preference.
To access the OSD (available anytime during the procedure when the Invisio ICN is in Active Mode):
- Depress and hold the distal and proximal remote buttons for two seconds.
To configure the remote buttons and system settings:
1. Depress the proximal button to display options, cycling through the various choices.
2. Depress the distal button to select a specific option.
3. No other buttons are active during programming.
4. The Invisio ICN System will remember the last confirmed remote button settings when the power is
disconnected from the Invisio IDC Digital Controller.
Section 5.6 OSD Options and Navigation
The screen at left depicts the menu that is displayed upon accessing the OSD.
1. Remote Button #1 – distally located
a. Control (select one)—the system will display the available choices for the distally located remote
button to control: Enhancement, Accessory 1, or Accessory 2.
2. Remote Button #2 – middle button
a. This button is dedicated to Brightness.
- Allows the surgeon to increase the maximum overall brightness of the image.
- The control will increase the image brightness if there is sufficient reserve.
- The image brightness will increase from level 1 to 5 and then back to 1 in this mode.
3. Remote Button #3 – proximally located
a. Control (select one)—the system will display the available choices for the proximally located
remote button to control: Enhancement, Accessory 1, or Accessory 2.
4. Remote Button Choices (Options)
a. Enhancement
- Allows the surgeon to increase the enhancement of the image.
- The image will be enhanced from level 1 to 5 and then back to 1 in this mode.
5. Dual Accessory Control
a. A VCR, digital capture device, or video printer can be controlled when a remote button is assigned
to Accessory 1 or Accessory 2.
b. VCRs will RECORD/STOP with each button press.
c. Video Printers/Digital Capture Devices will CAPTURE/PRINT with each button press. These devices
must be set correctly according to their manufacturers instructions.
d. Connect the accessory cable (#1) from the rear of the Invisio IDC Digital Controller to a device.
e. Connect the accessory cable (#2) from the rear of the Invisio IDC Digital Controller to a device.
f. The remote buttons, if configured, will now control or trigger a peripheral device.
6. Pre-Set Levels
a. Brightness
The Invisio IDC Digital Controller utilizes proprietary digital image processing to provide a bright
image across the entire visual field.
- Enables the base level of brightness to be set from 1 to 5 and back to 1.
- Factory setting from ACMI is level 3.
- Brightness level can be adjusted from the remote buttons, if configured, or via the front panel of
the Invisio IDC Digital Controller.
b. Enhancement
The Invisio IDC Digital Controller utilizes proprietary digital image processing to increase image
details. The digital enhance setting allows the user to see fine details that were previously
indistinguishable from their surroundings.
- Enables the base level of enhancement to be set from 1 to 5 and back to 1.
- Factory setting from ACMI is level 3.
- Enhancement level can be adjusted from the remote buttons, if configured, or via the front panel
of the Invisio IDC Digital Controller
99-1089 REV 10
11

Section 5.0
(continued)
Section 5.7 Inspection
Before using your Invisio ICN Flexible CystoNephroscope, inspect the endoscope, control unit, and cabling
(including connectors). A malfunction of any of these components can degrade the video image.
Invisio ICN Flexible CystoNephroscope Assembly
1. Thoroughly dry the connector prior to insertion into the Invisio IDC Digital Controller.
2. Confirm that the optical window on the distal end of the Invisio ICN Flexible CystoNephroscope is
clean and free of condensation.
3. Check the cable connector for signs of moisture, contamination, or physical damage (e.g., broken
housing or chipped circuit board).
System
1. Plug the Invisio ICN Flexible CystoNephroscope into the receptacle on the Invisio IDC Digital
Controller.
2. Connect video output signal(s) to the monitor (see Invisio IDC Digital Controller Operations and
Technical Manual 80819).
3. Point the Invisio ICN Flexible CystoNephroscope at a nearby object to verify that you are obtaining a
clear, high quality, color image on the video monitor.
4. Verify that the Invisio ICN Flexible CystoNephroscope will focus on objects at close range (5 mm) as
well as further away (50 mm) from the endoscope. Check that the image brightness on the monitor
remains reasonably constant. If not, see Section 6.2 Troubleshooting: Video Image.
Monitors
1. The Invisio ICN Flexible CystoNephroscope and monitor should be electrically isolated from
electrocautery and other electrical medical equipment.
2. Verify that the electrical equipment is safely grounded. Test your electrical sockets for proper
grounding and check that all plugs contain a ground prong.
3. Check that the monitor termination switch, if present, is set to 75 ΩON. (If no number is shown,
switch the monitor termination switch back and forth until you obtain the best picture.) If two or more
monitors are being used, only terminate the last connected monitor.
4. Make sure all of the cable connections are correct (see Invisio IDC Digital Controller Operations and
Technical Manual 80819).
Section 6.0
Troubleshooting Guide
Section 6.1 Troubleshooting: Invisio ICN Flexible CystoNephroscope
1. Accessory does not pass smoothly.
a) Accessory is bent or kinked; tip is sharp.
- Use new accessory.
b) Working channel is obstructed.
- Thoroughly clean channel of debris.
c) Instrument shaft is damaged.
- Inspect entire shaft for indentations, kinks, or other unusual wear that may indicate internal damage.
If damaged, return to ACMI for repair.
d) Friction has built up between working channel and accessory.
- Use a drop of lubricant (REF GLIDE) on the accessory or in the working channel to reduce friction.
2. Accessory cannot be inserted.
a) Accessory is too large.
- Be sure accessory is appropriate size for channel. See Section 3.2.
b) See #1d above.
c) Accessory is “hung up” on working channel diaphragm.
- Push through seal gently with short strokes.
d) Accessory jaws are not closed completely.
- Close accessory jaws before insertion.
3. Accessory cannot be passed while instrument is deflected.
a) Be sure tip is not deflected.
- Gradually withdraw scope to straighten tip until accessory passes.
4. Accessories do not operate smoothly.
a) Inspect accessory.
- Thoroughly clean; or, if damaged, discard and replace.
5. Articulation is not smooth or does not operate at all.
a) Listen for grinding noise when activating the deflection mechanism(s). If grinding noise is heard,
contact ACMI Customer Service for a replacement or to arrange for service.
12
99-1089 REV 10

Section 6.0
(continued)
6. Remote buttons are not activating a peripheral device.
a) Check OSD configuration (see Sections 5.4, 5.5, and 5.6 for more information).
b) Check remote accessory cable connections from the Invisio IDC Digital Controller to the peripheral
device.
c) Check that the peripheral device is configured correctly to handle the signal from the Invisio IDC Digital
Controller (see manufacturer’s instructions).
7. Remote buttons are not activating a system setting (i.e., Brightness or Enhancement)
a) Check OSD configuration (see Sections 5.4, 5.5, and 5.6 for more information).
Section 6.2 Troubleshooting: Video Image
1. Picture is grainy; image “whites out” easily when scope is close to tissue. Colors are slightly off.
a) Un-terminated monitor.
- Set termination switch at back of monitor to 75 ohm ON or termination position.
2. Periphery of image is cloudy or hazy. Colors are washed out. Spots appear on screen.
a) Dirty optics.
- Clean the distal tip of the Invisio ICN with a cotton swab and alcohol.
- Apply anti-fog agent if desired.
b) Leaking, nicked, or damaged scope.
- Inspect distal end of scope optics.
3. Picture is dark, grainy.
a) Insufficient light reaching subject.
- Inspect the distal tip of the Invisio ICN Flexible CystoNephroscope for dirt or other foreign
materials on the outer lens (see Section 4.2).
b) Improperly adjusted or double-terminated monitor or video printer.
- See monitor’s instruction manual and/or Section 5.7.
c) Increase brightness on front of controller or from the remote buttons on the Invisio ICN
CystoNephroscope.
4. No picture generated.
a) Broken video cable.
- Check that connecting ends of all cables are intact. Look for slices or other visual damage to the
cable. Replace cable as appropriate.
- Direct connect a cable from the Invisio IDC Digital Controller to the input of the monitor.
b) Lack of power to a system component.
- Ensure that all systems are plugged in and all accessories are switched to ON.
c) Off-line VCR or other peripheral device.
- Set VCR input switch to “line” position and/or refer to your VCR’s instruction manual.
d) Other components not in proper channel (e.g., the monitor may be in line “B” while hookup is in line
“A”).
- Connect components correctly.
5. Picture color is incorrect.
a) Improperly adjusted color.
- Check the monitor’s chroma and phase settings.
- Check the printer’s color and phase settings.
6. Picture is too dark.
a) Insufficient brightness.
- Increase brightness on front of controller or from the remote buttons on the Invisio ICN
CystoNephroscope.
b) Damaged Invisio ICN CystoNephroscope.
- Check condition of Invisio ICN CystoNephroscope.
c) Incorrect contrast or brightness settings on monitor or printer.
- Adjust contrast and/or brightness on monitor and/or printer.
7. Picture is too bright.
a) Excessive light intensity.
- Decrease brightness on front of controller or from the remote buttons on the Invisio ICN
CystoNephroscope.
b) Un-terminated monitor or secondary monitor cable.
- See Symptom 1, “Picture is grainy,” above.
8. Noise or “snow” appears on screen when no electrocautery is in use.
a) Excessive enhancement.
- Decrease enhancement level to low or off.
b) Faulty video cables.
- Check video cables and replace as necessary.
99-1089 REV 10
13

Section 6.0
(continued)
9. Noise or “snow” appears on screen when using electrocautery.
a) Dirty contacts in Invisio ICN CystoNephroscope receptacle (front panel).
- Clean contacts with ACMI Connector Maintenance Kit (
b) Interference from electrocautery unit.
- Plug electrocautery equipment into separate electrical outlet.
c) Interference from electrocautery cable.
- Separate video cable from electrocautery cable.
d) Poor video cable connection(s).
- Check all video cables for good condition and secure connection.
10. Picture is intermittent.
a) Wet Invisio ICN CystoNephroscope connector.
- Check cable-to-Invisio IDC Digital Controller connection.
- Shake and air-dry connection.
b) Dirty Invisio ICN connector contacts
- Clean contacts with ACMI Connector Maintenance Kit (
c) Failing Invisio ICN CystoNephroscope cable.
- Contact ACMI Customer Service.
11. Intermittent picture accompanied by green or unusual coloring.
a) Wet Invisio ICN CystoNephroscope connector.
- Check cable-to-Invisio IDC Digital Controller connection. Shake and air-dry connection.
b) Bad Invisio ICN CystoNephroscope cable.
- Contact ACMI Customer Service.
• For all other problems or when a recommended corrective action does not correct the identified
problem, contact ACMI Customer Service at (888) 524-7266 or (262) 639-7205.
REF MV-10454 or MV-10455).
REF MV-10454 or MV-10455).
Section 7.0
General Care
Like many precision surgical devices, the Invisio ICN Flexible CystoNephroscope and Invisio IDC Digital
Controller are delicate and should be handled with care. Follow the suggestions below to prolong your
system’s life and avoid repairs:
• Never remove the Invisio ICN Flexible CystoNephroscope cable from the Invisio IDC Digital Controller
by pulling on the cable itself—always pull from the connector body to remove.
• Coil the Invisio ICN Flexible CystoNephroscope cable loosely—never sharply bend, kink, strain, or
crush the cable. Exercise care when handling the connector at the end of the Invisio ICN Flexible
CystoNephroscope cable. Damage may occur if the connector is dropped or forced into the receptacle.
• Keep unprotected instruments, cables, and Invisio IDC Digital Controllers separate from one another.
Do not stack system components.
• Pick up and carry instruments or system components separately, not in groups.
• Never insert a wet cable connector into the Invisio IDC Digital Controller receptacle.
• Use ACMI Connector Maintenance Kits (REF MV-10454, MV-10455) for proper care of connector and
cable receptacle.
Section 8.0
Maintenance
The following cleaning, disinfection, and sterilization procedures are recommended by ACMI. Use of
any procedures not expressly recommended by ACMI may adversely affect or damage ACMI devices
and may void ACMI’s Limited Express Warranty.
Follow all applicable bloodborne pathogen procedures as indicated by OSHA and/or your hospital
requirements when cleaning, disinfecting, and sterilizing instruments and accessories.
WARNING: Always turn off the power on the Invisio IDC Digital Controller and disconnect the Invisio
ICN Flexible CystoNephroscope before cleaning.
14
99-1089 REV 10

Section 9.0
(continued)
Section 8.1 Cleaning - Invisio ICN Flexible CystoNephroscope
Clean the Invisio ICN Flexible CystoNephroscope and optics after each use with an enzymatic cleaner
formulated to dissolve proteinaceous material.
1. Separate any assembled components.
2. Rinse the scope with warm (38–49 °C) water on all exterior surfaces.
3. Flush the working channel with warm (38–49 °C) water using a syringe.
4. Prepare enzymatic detergent solution per the manufacturer’s written instructions.
5. Immerse the scope in the enzymatic solution. Wash the exterior of the scope with the enzymatic
detergent solution and a soft brush.
6. Allow the scope to soak in the enzymatic solution for 10 minutes.
7. Brush the exterior of the scope thoroughly in the enzymatic solution. Pay special attention to the area
around the deflection handle and the function buttons.
8. Clean the interior working channel with enzymatic detergent solution using a working channel
cleaning brush (ACMI
cleaned through to the distal end of the scope.
9. Flush each working channel inlet with a minimum of 50 cc of enzymatic detergent solution using a
syringe.
10. Rinse the exterior of the scope by immersing it in warm water.
11. Rinse each inlet with 50 mL minimum of warm water.
12. Purge air through each inlet using a 50 cc syringe.
13. Repeat steps 6–11 at least two times.
14. Clean the lens with a cotton swab moistened with 70% isopropyl alcohol. Avoid scratching the lens
with a hard object.
15. Dry the exterior of scope using a sterile, lint-free towel or air jet.
REF CB-560: 5 Fr, 60 cm long). Ensure both working channel and inlets are
Section 8.2 Cleaning the Optics on the Invisio ICN Flexible CystoNephroscope
CAUTION: Do not attempt to open the Invisio ICN Flexible CystoNephroscope. Opening these sealed
assemblies affects the Invisio ICN’s moisture seals and will void your warranty.
Section 8.3 Cleaning the Invisio ICN Connector and Receptacle
ACMI recommends the use of the Connector Maintenance Kits (REF MV-10454, MV-10455). Follow the
cleaning instructions provided with these kits.
Section 8.4 Cleaning the Invisio IDC Digital Controller
CAUTION: Never immerse the Invisio IDC Digital Controller in any solution.
If dirt or liquid gets on the Invisio IDC Digital Controller, wipe with a damp cloth or sponge. Dry with a towel
after cleaning it. Depending on use, clean at least once a week.
Section 8.5 Disinfection - Invisio ICN Flexible CystoNephroscope
1. Keep all components disassembled.
2. After cleaning and inspection, completely immerse the Invisio ICN Flexible CystoNephroscope and
cable in a 2.4% activated alkaline glutaraldehyde solution in a plastic or enamel basin while keeping
the shaft in a soft curl.
3. Fill the working channel with the solution, using a syringe.
4. Soak for a minimum of 45 minutes (not to exceed 60 minutes).
5. Thoroughly rinse the outside of the Invisio ICN Flexible CystoNephroscope and the cable and flush the
working channel three times with sterile water, using a syringe.
6. Wipe the Invisio ICN Flexible CystoNephroscope including cable and cable connector paddle dry using
a sterile towel. The cable connector paddle must be completely dry before connecting to the
controller.
7. DO NOT DISINFECT THE IDC DIGITAL CONTROLLER.
Section 8.6 Autoclave Sterilization
CAUTION: Never steam sterilize the Invisio ICN Flexible CystoNephroscope or IDC Digital Controller.
Exposing the Invisio ICN Flexible CystoNephroscope or IDC Digital Controller to temperatures greater
than 63 °C (145 °F) will cause irreparable damage.
99-1089 REV 10
15

Section 8.0
(continued)
Section 8.7 Ethylene Oxide Sterilization
The Invisio ICN Flexible CystoNephroscope can be sterilized in an ethylene oxide (EO) sterilizer.
1. Thoroughly clean, rinse, and dry prior to sterilization.
2. The instrument can be placed in an ethylene oxide (EO) sterilizer controlled within the following
parameters:
EO (100%) Temp: °F (°C) Rel. Humidity Vacuum Time Gas Conc. Exposure
131 (55) ≥ 35% 2–3 psia ≥ 60 minutes 725–750 mg/l ≥ 60 minutes
3. After EO exposure, aerate for a minimum of 12 hours at 122 °F (50 °C) in a mechanical aerator.
Preconditioning
Section 8.8 STERIS SYSTEM 1™ Sterilization
The Invisio ICN Flexible CystoNephroscope is compatible with the STERIS SYSTEM 1 when used according
to the STERIS written instructions. It is important to use the C1140 Covered Flexible Processing Tray/
Container and Quick Connect QCC1671 to process the CystoNephroscope.
Section 8.9 STERRAD® Sterilization
In countries where STERRAD is approved for use with flexible endoscopes, the Invisio ICN Flexible
CystoNephroscope has been validated for both sterilization and material compatibility.
Section 9.0
Storage
To pack the Invisio ICN Flexible CystoNephroscope System components for storage, disconnect the Invisio
IDC Digital Controller from the Invisio ICN Flexible CystoNephroscope and the monitor. Separate the
power cable from the Invisio IDC Digital Controller, coil it loosely, and place it into the storage container.
Store the system and components in a clean, dry, well-ventilated environment.
Take special care to provide the Invisio ICN Flexible CystoNephroscope assembly with an extra level of
protection during storage; the distal tip, shaft, cable, and connector are fragile. Never strain, crush, kink,
or sharply bend the power cable, the Invisio ICN Flexible CystoNephroscope cable, or shaft. Exercise care
when handling the connector at the end of the Invisio ICN Flexible CystoNephroscope cable—damage may
occur if the connector is dropped.
Store the Invisio ICN Flexible CystoNephroscope in a protected vented tray; do not store in shipping case.
Be certain instruments are completely dry prior to storage. Keep instruments separate from one another to
avoid damage. Keep the shaft in the non-deflected (straight) position when storing.
Section 10.0
Theory of Operation
Section 10.1 General
The system consists of two major components: the Invisio ICN Flexible CystoNephroscope and the Invisio
IDC Digital Controller. The Invisio ICN Flexible CystoNephroscope contains a single image sensor placed
within the distal tip of the endoscope upon which a scene is imaged. The image sensor consists of an active
area that converts light into electrical charges that form the basis for generating an image. The parts of the
active area are often referred to as “pixels,” short for “picture elements.”
Refer to Invisio IDC Digital Controller Operations and Technical Manual 80819 for more information.
16
™ STERIS SYSTEM 1 is a U.S. trademark of STERIS Corporation
® STERRAD is a registered trademark of Johnson & Johnson Medical, Inc.
99-1089 REV 10
 Loading...
Loading...