ACMI DUR Invisio User and service manual
ref NTSC/PAL/VGA: DUr-D, DUr-DBA
DUR®-Digital Invisio® Flexible Ureteroscope/Choledochoscope
Operations and Technical Manual
ACMI Corporation
136 Turnpike Road • Southborough, MA 01772-2104 • USA
(888) 524-7266 • www.gyrusacmi.com
Authorized European Representative: MDCI, Ltd.
Arundel House • 1 Liverpool Gardens • Worthing, West Sussex BN11 1SL • United Kingdom
® Registered trademark of ACMI Corporation or its Subsidiaries in the U.S. and/or other countries of the world
© 2006 ACMI Corporation, All Rights Reserved U.S. Patent Nos. 5,938,588; 6,485,411; 6,749,560; 6,780,151; other patents pending
99-1091 REV B 0906

Table of Contents
Repair/Return/Warranty .................................................................................................3
Warnings and Cautions ...................................................................................................4
Section 1.0 Unpacking and Initial Inspection ...............................................................5
Section 2.0 General Description and Intended Use ......................................................5
Section 3.0 Description of Components .......................................................................6
3.1 The Instrument & Accessories ........................................................................ 6
3.2 Technical Specifications ..................................................................................7
Section 4.0 Operation ...................................................................................................8
4.1 Inspection of the Instrument ...........................................................................8
4.2 Optical Field Inspection ...................................................................................8
4.3 Deflection Controls ..........................................................................................8
4.4 Leakage Tester ................................................................................................ 9
4.5 AUTOSEAL™ Feature ......................................................................................9
4.6 Rotating Working Channel/Irrigation Port .......................................................9
4.7 Strain Relief .................................................................................................. 10
4.8 Cable Compensation .....................................................................................10
Section 5.0 Procedure ................................................................................................ 10
5.1 Working Channel ...........................................................................................11
5.2 White Balancing ............................................................................................11
5.3 Focusing ........................................................................................................11
5.4 Remote Head Button Setup ...........................................................................11
5.5 On-Screen-Display (OSD) System .................................................................11
5.6 OSD Options and Navigation .........................................................................12
5.7 Inspection .....................................................................................................13
Section 6.0 Troubleshooting Guide ............................................................................. 13
6.1 Troubleshooting the Instrument .....................................................................13
6.2 Troubleshooting: Video Image ....................................................................... 14
Section 7.0 General Care ...........................................................................................16
Section 8.0 Maintenance............................................................................................16
8.1 Cleaning ........................................................................................................16
8.2 Cleaning the Optics on the Instrument ..........................................................17
8.3 Cleaning the Instrument Connector and Receptacle .....................................17
8.4 Cleaning the IDC Invisio Digital Controller ....................................................17
8.5 Disinfection ...................................................................................................17
8.6 Autoclave Sterilization ..................................................................................17
8.7 Ethylene Oxide Sterilization ...........................................................................17
8.8 STERIS SYSTEM 1™ Sterilization .................................................................18
8.9 STERRAD® Sterilization ................................................................................. 18
Section 9.0 Storage .................................................................................................... 18
Section 10.0 Theory of Operation .................................................................................18
2 99-1091 REV B

DUR-Digital Invisio Flexible Ureteroscope/Choledochoscope • Operations and Technical Manual
Repair/Return/Warranty
Repair/Return
All returns for credit must have prior written authorization. All medical devices returned to ACMI
for any reason must be 1) disassembled, 2) cleaned, and 3) high-level disinfected or sterilized in
accordance with the product’s instructions for use or labeling, and shipped in accordance with
ACMI’s return procedures (available upon request) and all applicable regulations. To obtain a return
authorization number, return addresses, and instructions, please call toll-free (888) 524-7266
Limited Express Warranty
SHOULD THE PRODUCT BECOME INOPERABLE, DURING NORMAL AND PROPER USE IN
ACCORDANCE WITH APPLICABLE INSTRUCTIONS, WITHIN THE TIME FRAME SPECIFIED BELOW
FROM THE DATE OF SHIPMENT, ACMI WILL REPAIR OR REPLACE THE PRODUCT, AT ITS SOLE
OPTION, AT NO CHARGE. ACMI MAKES NO OTHER WARRANTIES WITH RESPECT TO THE
PRODUCTS AND EXPRESSLY DISCLAIMS ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, AS TO
MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER MATTER. IN NO EVENT
SHALL ACMI BE LIABLE FOR ANY CONSEQUENTIAL DAMAGES. IN NO EVENT SHALL ACMI BE
LIABLE FOR ANY BREACH OF WARRANTY IN ANY AMOUNT EXCEEDING THE PURCHASE PRICE OF
THE PRODUCT.
This Warranty runs only to the original user and may be voided if the product(s) are serviced or
repaired by anyone other than ACMI or an organization duly authorized by ACMI for such purpose.
DUR-Digital Invisio Flexible Ureteroscope (excluding cable) ................................ 1 year
DUR-Digital Invisio Flexible Ureteroscope cable only ........................................... 1 year
Accessories .........................................................................................................90 days
99-1091 REV B 3

Warnings and Cautions
The following warnings and cautions apply to the use, care, and/or maintenance of the ACMI®
DUR-Digital Invisio Flexible Ureteroscope System. Failure to comply with, or abide by, any warning or
caution set forth in this manual will void the video system’s Limited Express Warranty.
Warnings
(indicate that a danger to life or health can result from misusing the equipment)
1. SERVICE WARNING AND DISCLAIMER NOTICE: It is recommended that the medical equipment
for which these documents apply be returned to ACMI for servicing or repair. Service or repair
provided by any party other than ACMI or an ACMI-authorized repair facility may result in the
user and/or repair facility being liable and responsible for damages, including patient or user
injuries, arising from such servicing or repair, and any such servicing or repair shall void the
manufacturer’s Limited Express Warranty, if any, applicable to such medical equipment.
2. To prevent electric shock and breakage of seals, do not remove covers on the instrument or IDC
Invisio Digital Controller as a potential electric shock hazard exists. Refer all repairs to ACMIauthorized service personnel.
3. Study this manual and other labeling thoroughly for safe handling and storage. Misuse of the
equipment can cause injury to the patient and could have an adverse effect on the procedure
being performed. Do not drop equipment, or allow it to be struck by other objects.
4. Procedures should be performed only by persons with adequate training and preparation. Consult
the medical literature regarding techniques, complications, and hazards prior to any procedure.
5. Do not use electromedical energy sources in the presence of flammable detergents, anesthetics,
nitrous oxide (N2O), or oxygen.
6. Consult the operating manuals of all electromedical energy sources used with endoscopic
instruments for appropriate instructions, warnings, and cautions prior to use. Such sources of
energy include electrical, electrohydraulic, electrosurgical, heat, hydraulic, laser, light, pressure,
sound, ultrasound, and vacuum.
7. Make no repairs to any instrument or IDC Invisio Digital Controller. Personal injury or damage to
units may result. Refer units to ACMI-authorized service personnel for repair.
8. Never look directly into the light emitted from the instrument. Damage to the eyes can result.
9. Keep the distal tip of any electrode, probe, laser fiber, or other ancillary device in the field of view
at all times when active.
10. Use only those lubricants specified in the labeling.
11. Prior to use, examine each electrode and its insulation for damage; do not use if damaged.
12. Follow the disinfectant or sterilizer manufacturer’s recommended procedures and cautions.
13. Do not use disinfectant solutions that contain long-life surfactants; such solutions can leave
conductive residues.
14. Do not use an instrument that fails to meet the criteria stated in the labeling or that has been
damaged.
15. When instruments from different manufacturers are used together, verify that any isolation or
grounding is not violated.
16. Follow the labeling instructions regarding the disposal or reuse of accessories. Reuse of
disposable accessories could compromise patient safety.
Cautions
(indicate that equipment or other property may be damaged or may malfunction by misuse)
1. Pay close attention to the care, cleaning, disinfection, and sterilization instructions in this manual.
Any deviation can cause damage and void ACMI’s Limited Express Warranty. Do not steam
sterilize the instrument or IDC Invisio Digital Controller.
2. To ensure continued satisfactory performance, perform the prescribed inspections and
operational tests as recommended.
3. Thoroughly check all electrical cables and plugs before each use and replace any which are
damaged or excessively worn. Do not use if damage is suspected or discovered.
4 99-1091 REV B

DUR-Digital Invisio Flexible Ureteroscope/Choledochoscope • Operations and Technical Manual
4. Check the outer surface of the flexible shaft prior to each use to make certain that the instrument
is free of any cuts, holes, rough surfaces, sharp edges, or protrusions. Do not use if damage is
suspected or discovered.
5. Do not insert a wet connector into the IDC Invisio Digital Controller receptacle, as poor video
performance and/or damage to the system may result.
6. These instruments contain no user-serviceable parts. Refer all repairs to ACMI-authorized service
personnel.
7. Connect electromedical energy source power cables only to properly wired grounding
receptacles.
8. The instrument is listed by CSA as meeting Medical and Dental Equipment Standard UL 2601 and
CSA Standard 22.2 No. 601 and IEC-60601.
9. Prior to use of a cardiac defibrillator, remove endoscopic instruments from the patient. Failure to
remove an endoscopic instrument from a patient during use of a cardiac defibrillator could result
in damage to the instrument due to the discharge of the cardiac defibrillator.
10. Testing to ensure that chassis leakage does not exceed the allowable levels per the appropriate
standard should be performed at least once each year.
11. Do not adjust electronic circuitry. Electronic circuitry is set at the factory and no further
adjustment is necessary.
12. Never remove the instrument from the IDC Invisio Digital Controller by pulling on the cable.
Always pull the insertion (connector) body to remove the cable from the IDC Digital Controller.
13. U.S. Federal law restricts this device to sale by or on the order of a physician.
Section 1.0 Unpacking and Initial Inspection
Upon receipt, examine the shipping carton and its contents for signs of damage. Check for rattling
or loose material inside. Examine the instrument, IDC Invisio Digital Controller (sold separately), and
accessories for damage. Do not use a damaged product—contact ACMI Customer Service at (888)
524-7266.
It is very important that upon unpacking the equipment the proper video format selection is made:
PAL, VGA (Progressive Scan), or NTSC.
See IDC Invisio Digital Controller Operations and Technical Manual for more detailed instructions.
Section 2.0 General Description and Intended Use
The instrument system consists of a Digital Flexible Ureteroscope with an integrated cable,
accessories, an IDC Invisio Digital Controller, and all required video connection cables.
The ACMI instrument system (which includes the DUR-Digital Invisio Flexible Ureteroscope,
Choledochoscope, and IDC Invisio Digital Controller) is intended for use to examine body cavities,
hollow organs and canals in the body, in the urinary tract, and can be used percutaneously to examine
the interior of the kidney; and using additional accessories, can be used to perform various diagnostic
and therapeutic procedures.
The DUR-D System is also indicated for the examination of bile ducts, and using additional
accessories, to perform various diagnostic and therapeutic procedures during cholecystectomy.
99-1091 REV B 5

Section 3.0 Description of Components
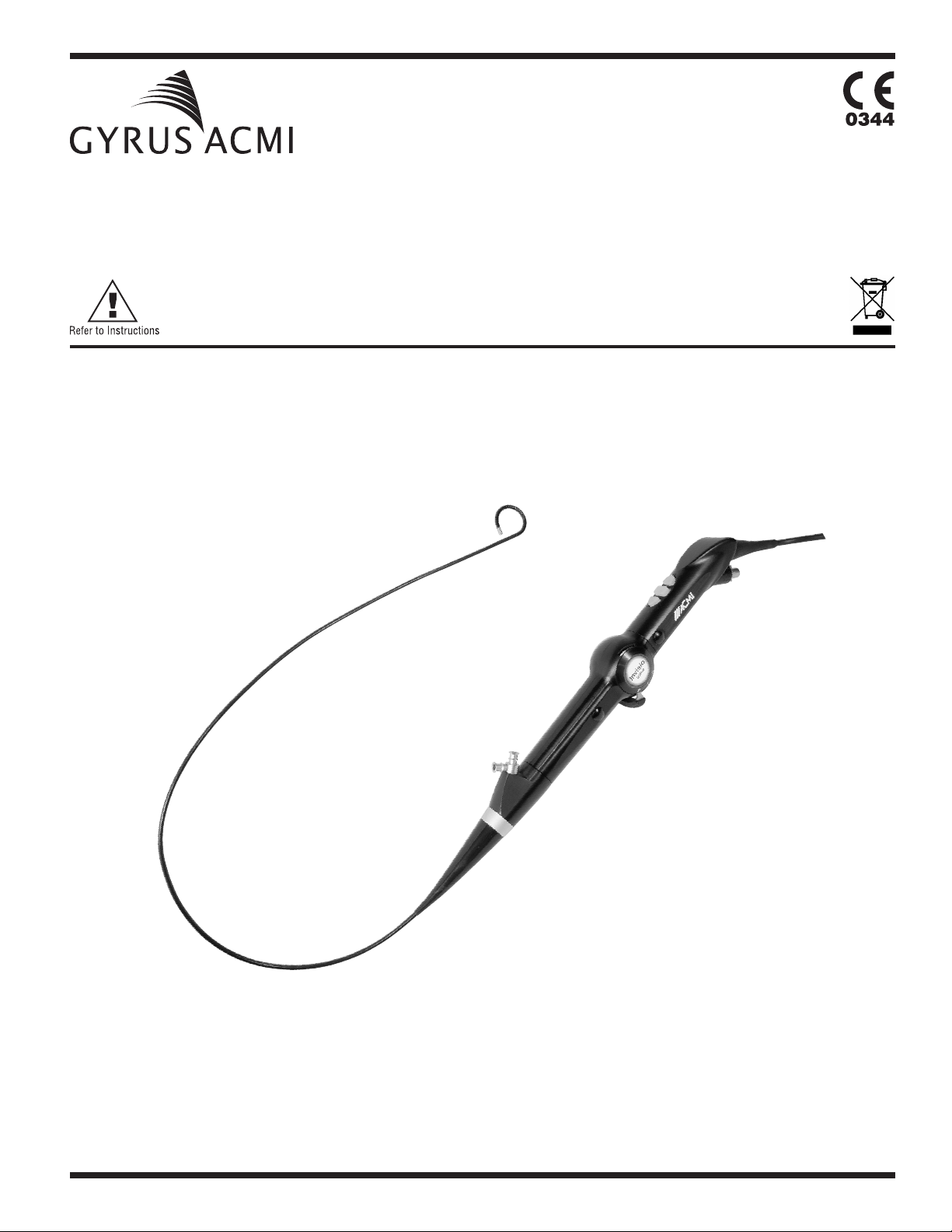
DUR-Digital Invisio Flexible Ureteroscope
The distal tip of the instrument contains a digital imaging sensor that converts light energy into an
4
3
8
2
6
1
7
5
10
6
9
electrical signal for the production of an intra-operative video image that is displayed on a monitor. The
three remote push buttons on the Ureteroscope allow fingertip control of DUR-D System functions, as
well as control of external devices and accessories.
Figure Identifications
1. Deflection Control Levers UP and DOWN
2. Programmable Remote Control Buttons
3. Working Channel
6
4. Irrigation Port
5. Leak Test Valve
6. Strain Reliefs
7. Flexible Shaft
8. Deflection Section
9. Insertion (Connector)
10. Card Edge Printed Circuit Board Plug
3.1 The Instrument & Accessories
DUR-D Invisio Flexible Ureteroscope, 3.1mm, 65cm, 3.6Fr channel
DUR-DBA Invisio Flexible Ureteroscope, backward ar ticulating,3.1mm, 65cm, 3.6Fr channel
DUR-D and DUR-DBA are compatible with IDC-1000 and IDC-1500 controllers. For appropriate
controller software upgrades, contact ACMI Customer Service or your local ACMI representative.
Accessories
Remote Printer Cable, Sony Printers MV-10350
Remote Printer Cable, Toshiba Printers MV-10351
BNC Cables, 4 ft. (2/pkg) MV-14024
BNC Cables, 12 ft. (2/pkg) MV-9144
BNC Cables, 40 ft. (2/pkg) MV-9144-40
Y/C (S-Video) Cables, 4 ft. (2/pkg) 103017-1
Y/C (S-Video) Cables, 12 ft. (2/pkg) MV-9162
Y/C (S-Video) Cables, 30 ft. (2/pkg) MV-9162-30
RGB (Component) Cable, 6 ft. IDC-0110
VGA (Progressive Scan) Cable, 6 ft. IDC-0100
Power Cables, 4 ft., 120 V (2/pkg) MV-10428
Connector Maintenance Kit, small MV-10454
Connector Maintenance Kit, large MV-10455
6 99-1091 REV B
 Loading...
Loading...