Page 1
3M™ Attest™ Steam
Chemical Integrator
Technical Information
STEAM
Dynamics of Steam Sterilization
Steam sterilization has been used for over 100 years. Decades of research have
shown that the ecacy of a steam sterilization process is the function of three
basic parameters: time, temperature and the presence of saturated steam. All
three are critical process variables for eective steam sterilization.
The importance of saturated steam is demonstrated when dry heat sterilization
is compared with steam sterilization. The use of steam allows faster sterilization
than dry heat. For example, dry heat sterilization requires a sterilization time of
60 minutes at 320°F (160°C), while steam sterilization at the same temperature
would take less than a minute.1 Clearly, steam quickens the kill time of living
organisms by many orders of magnitude and is generally preferable to dry heat.
Page 2
2
3M™ Attest™ Steam Chemical Integrator Technical Information
Once a saturated steam environment is obtained, the
independent variables of time and temperature can be
determined by the following formula:
t = Fo × 10
Where
t = time for 100% kill at temperature T
(2 50-T )/Z
2
T = processing temperature (0°F)
Fo = kill time for Geobacillus
stearothermophilus with a z-value of
18°F (10°C) and D-value of 1 minute at 250˚F
(121˚C)
z = rise in temperature required to increase
the rate of kill by a factor of 10 (usually
about 18˚F (10˚C))
Interpretation of this formula shows that the
relationship of processing time (t) versus temperature
(T) can be plotted as a logarithmic function. Expressed
dierently, it means that a small uctuation in the
temperature results in a large change in the actual
processing time required for 100% kill. Figure 1 shows
the thermal death time at dierent temperatures for
1 million live spores of Geobacillus stearothermophilus.3
This curve can be expressed mathematically by the
following formula which shows that it takes 12 minutes
to kill 1 million living spores in a 250°F (121°C) steam
sterilization cycle.
t = (12)10
(250-T)/18
Where
Fo = 12 min for G. stearothermophilus
z = 18°F (10°C) for G. stearothermophilus
In order to show the high sensitivity of kill time to
temperature, the above formula can be solved for
247°F (119°C).
t = (12)10
t = (12)10
(250-247)/18
(0.167)
= (12)(1.47)
t = 17.6 minutes
In theory, therefore, if the inside temperature of a
sterilizer were actually operating at 247°F (119°C)
instead of 250°F (121°C), a time of 17.6 minutes
would be required to kill the 1 million spores of G.
stearothermophilus at 247°F (119°C) versus the 12
minutes needed to kill the spores at 250°F (121°C).
This interdependence of time and temperature (in
saturated steam) is an important relationship which
should be understood by all personnel responsible
for providing sterility assurance for steam sterilized
items. Consider the ramications if a sterilizer was
inadvertently set at a processing temperature at
247°F (119°C) instead of 250°F (121°C). Or, if the load
was processed at 247°F (119°C) as a result of a minor
malfunction of the sterilizer (e.g., air pocket or small air
leak), a slight calibration error or a natural drift in the
temperature monitoring system, incorrect loading or
packaging.
Because even small decreases in temperature during
steam sterilization may signicantly increase the time
necessary for assurance of sterility, an accurate means
of monitoring internal sterilizer and pack conditions
are essential.
Page 3
3M™ Attest™ Steam Chemical Integrator Technical Information
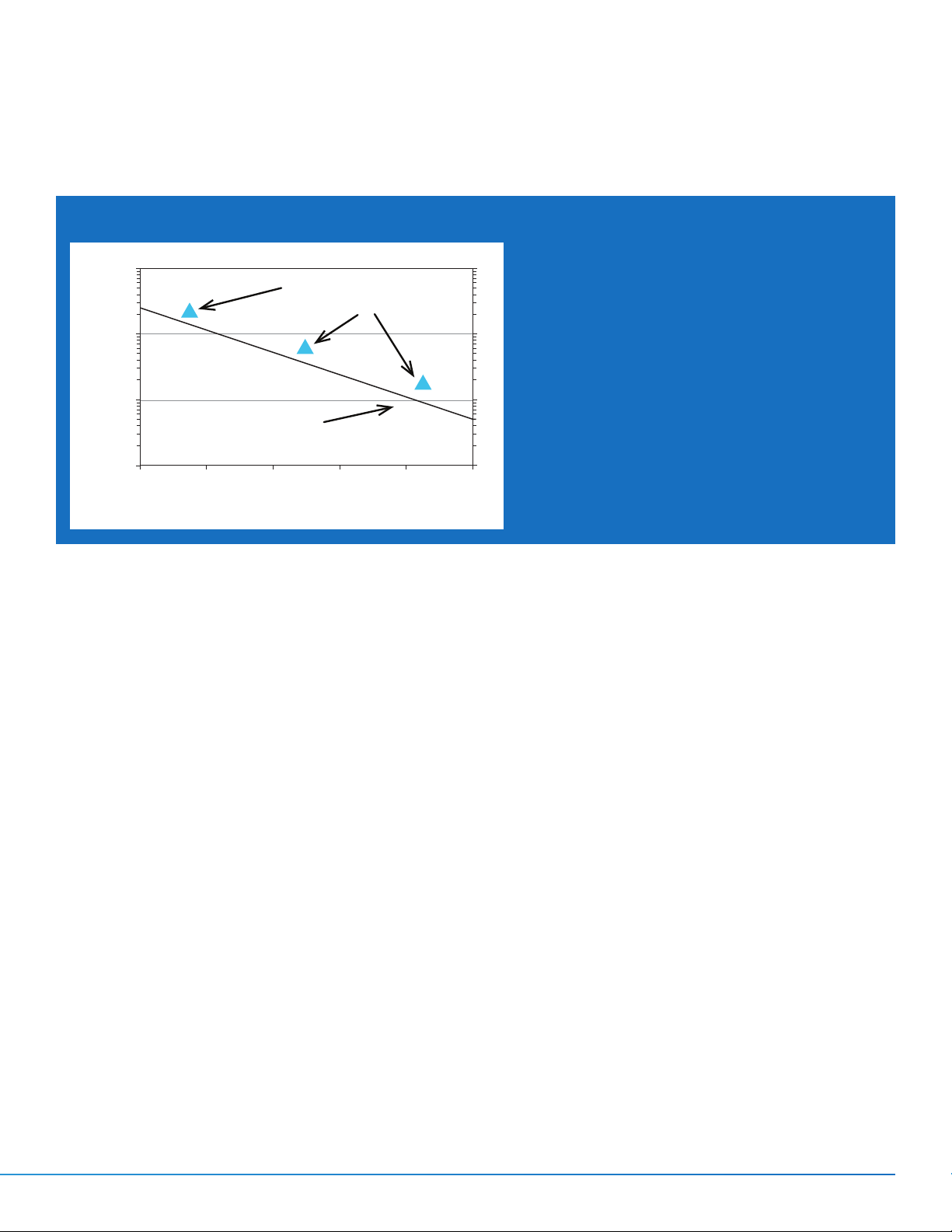
Integrating Indicator vs. Biological Death Curve
3
100.00
10.0
1.0
Time (min.)
Theoretical G. stearothermophilus
Thermal Death Time
0.1
118 122 126 130 134 138
Three Typical Stated Values
™
Attest™ Steam
for 3M
Chemical Integrators
Temp (°C)
Pack and Load Control
The dynamics of steam demonstrate the need for
accurate monitoring of internal sterilization conditions.
Pack control is the use of chemical indicators for the
internal monitoring of packs, trays, containers, and peel
pouches. Internal chemical indicators should be used
inside each type of packaging to address the potential
for interference with proper steam sterilization
conditions in all of these types of packaging.
Several problems can occur in the packaging and
loading of individual packs that can inhibit air removal
and steam penetration which leads to a lower
temperature. Packing problems include:
• Incorrect packaging or container system chosen
for the cycle parameters;
• Incorrect preparation of the container for use
(i.e., lters and valves or inappropriate bottom tray);
• Placing a folded peel pouch inside another
peel pouch;
• Placing a peel pouch inside of an instrument tray
or container system (if not recommended by the
manufacturer);
• Preparing textile packs that are too dense to sterilize
in the cycle parameters chosen;
4,5,6
Figure 1. Graph comparing three
typical Stated Values of 3M™ Attest™
Steam Chemical Integrators with the
theoretical death curve of Geobacillus
stearothermophilus spores.
• Over loading the individual packaging or container
system chosen (an over weight package).
Loading problems include:
• Stacking container systems (if not recommended
by the manufacturer);
• Laying peel pouches at or on top of each other
instead of on edge;
• Improperly placing peel pouches on edge (plastic
sides not facing all in one direction);
• Turning instrument trays on edge;
• Laying fabric packs or basins at;
• Placing packages too close to each other impeding
air removal and sterilant penetration around and
through the load;
• Rigid containers systems loaded above wrapped
or pouched items.
Malfunctioning equipment can also result in
insucient sterilization conditions inside of
packaging as the result of:
• Incomplete air removal;
• Inadequate cycle temperature;
Page 4

4
3M™ Attest™ Steam Chemical Integrator Technical Information
• Insucient time at temperature;
• Poor steam quality and quantity.
As discussed above, small reductions in time at
temperature can reduce the margin of safety with
steam processing. Problems that limit air removal or
steam penetration in individual packs may reduce
the eective time at temperature. Type 5 Integrating
Integrators that meet ISO 11140-1:2014 Sterilization
of healthcare products-Chemical Indicators-Part 1:
General requirements used inside each pack to monitor
time, temperature and steam exposure conditions can
provide the necessary sterilization assurance on
a pack-to-pack basis.
Load control is the process by which a load is
monitored and released based on the result of a
Biological Indicator (BI) in a process challenge device
(PCD). A BI PCD should be used, preferably every
day the sterilizer is used, for routine sterilizer ecacy
testing. BI PCDs are also recommended for sterilizer
qualication testing.
5 Integrating Indicator should be used to monitor each
implant load. The load should be quarantined until the
results of the BI testing are available.
loads, a PCD containing a Type 5 integrating indicator
or Type 6 emulating indicator may be used to release
the load.
Using a BI in every load (ELM) is considered best
practice. When you monitor every load with a BI you
provide the same level of care to each and every
patient served by the facility. Every load monitoring
allows only one load to be recalled, aecting fewer
departments, and protecting the reputation of the
department and the facility overall. It allows the CSD
Manager to streamline workow, simplify training and
reduce the opportunity for human error which leads to
consistent/standardized practice. Follow the process
for every load and reduce the risk of failing to properly
monitor an implant load.
7
4,5
A BI PCD that includes a Type
4,5
In non-implant
The chemical pellet melts and migrates as a dark color
along the paper wick. The migration is visible through a
green window marked ACCEPT or red window marked
REJECT; the extent of migration depends on steam,
time, and temperature. 3M™ Attest™ Steam Chemical
Integrators are Type 5 (Category i5) Integrating
Indicators as categorized by ISO 11140 1:2014.
This product comes with an extender strip axed to
one end of a 3M™ Attest™ Steam Chemical Integrator.
The axed 1243RE extender is a 17.8cm (7 in) long by
1.8cm (0.7 in) wide rigid strip. The axed 1243RES
extender is a 5.1cm (2 in) long by 1.8cm (0.7 in) wide
rigid strip. The extender serves as a handle to retrieve
processed integrators from inner packs.
Indications for Use
Outside the United States
Use 3M™ Attest™ Steam Chemical Integrators for pack
control monitoring of all 121–135ºC (250–275ºF) steam
sterilization cycles.
Inside the United States
The 3M™ Attest™ Steam Chemical Integrators are
designed to respond to all critical parameters over
a specied range of steam sterilization cycles. The
integrating indicator is intended to be placed in each
pack, pouch, container, tray or other containment
device to function as an independent monitor of
critical parameters for sterilization cycles. Please see
Instructions for Use for specic cycle indications for
use in the U.S.
Contraindications
None.
Product Description
3M™ Attest™ Steam Chemical Integrators 1243A,
1243B and 1243RE are chemical indicators consisting
of a paper wick and steam and temperature sensitive
chemical pellet contained in a paper/lm/foil laminate.
Precautions
Do not use 3M™ Attest™ Steam Chemical Integrators to
monitor dry heat, ethylene oxide, hydrogen peroxide,
or other low temperature sterilization processes.
Page 5

3M™ Attest™ Steam Chemical Integrator Technical Information
Clear Plastic
Display Window
Paper/Film Top Cover
Steam Penetration
1
Rate Controlling
Paper Wick Strip
2
Steam and Temperature
3
Sensitive Chemical Pellet
5
Technical Design
The 3M™ Attest™ Steam Chemical Integrator is made
of four functional components. These components are
arranged in a sandwich conguration held together
with a pressure-sensitive adhesive: (See above)
Steam penetration rate controlling paper/lm
1
top cover
Paper strip (for chemical wicking)
2
Steam and temperature sensitive chemical pellet
3
Aluminum foil bottom cover
4
The base of the 3M™ Attest™ Steam Chemical
Integrator is made of aluminum foil several mils
thick which acts as a moisture barrier against steam
penetration during sterilizing. A cavity embossed in the
foil holds the temperature and steam sensitive chemical
pellet. The pellet has a very high dry heat melting point
to ensure 3M™ Attest™ Steam Chemical Integrators
require the presence of steam in order to respond.
However, it is designed to melt at lower temperatures
Bottom Cover
4
when subjected to a steam environment. The top cover
of the 3M™ Attest™ Steam Chemical Integrator is a
paper/polymeric lm which allows steam to penetrate
at a certain rate. As steam penetrates the polymeric
cover lm, it lowers the melting point of the chemical
causing the tablet to begin melting.
When melting occurs, the liquid chemical is absorbed
by the paper wick and, as time elapses, moves along
the scale. The more the chemical melts, the farther the
color front advances towards the ACCEPT area of the
display window. This is in part a function of the movingfront technology. The rate at which the chemical pellet
melts is a function of the time, temperature, steam and
the inherent design of the 3M™ Attest™ Steam Chemical
Integrator. The combination of these factors provides
a rate of melting at various temperatures which closely
follows the spore death curve of G. stearothermophilus
(proven to be the best challenge in a steam sterilization
process) (see Figure 1).
Page 6

6
3M™ Attest™ Steam Chemical Integrator Technical Information
Chemical Indicator Types
3M™ Attest™ Steam Chemical Integrators meet the
requirements of ISO 11140-1:2014 for Type 5 Integrating
Indicators. These indicators are designed to monitor all
three of the critical variables of the steam sterilization
process (time, temperature and steam) across full range
of steam sterilization temperature use.
Third Party Testing
As part of our compliance process, 3M hired BSI, a
leading global independent product testing services
company, to conrm that 3M™ Attest™ Steam Chemical
Integrators meet the Type 5 Integrating Indicator
performance requirements of ISO 11140-1:2014.
Through rigorous product testing, BSI conrmed these
products meet the performance requirements of ISO
11140-1:2014. A copy of the BSI Kitemark™ Certication
is available upon request from 3M.
Performance Characteristics
The 3M™ Attest™ Steam Chemical Integrator has been
tested at various time and temperature intervals in
saturated steam in a test vessel (called a resistometer)
to determine compliance to the chemical indicator
standards listed in the Chemical Indicator Types
section above. To meet the Type 5 Integrating
Indicator performance standards, the 3M™ Attest™
Steam Chemical Integrator must have a response
that correlates to the performance of a BI at three
temperatures (121°C/250°F, 135°C/275°F, and one or
more equally spaced temperature points in the range of
121°C/250°F to 135°C/275°F, such as 128°C/263°F).7
These responses are called Stated Values. Stated
Values are “value or values of a critical process variable
at which the indicator is designed to reach its endpoint
as dened by the manufacturer.”7 In addition, the
Stated Value at 121°C/250°F must be >16.5 minutes and
the 135°C/275°F stated value must be >1.2 minutes.7
These are the most important Stated Values to ensure
that chemical indicators labeled for use in 132°C/270°F
do not change too quickly or inappropriately at lower
and higher temperatures (to ensure the performance
of the CI is consistent between all temperatures)
and to ensure that all temperatures correlate to the
performance of a BI. Furthermore, all of the ISO 11140-
1:2014 Type 5 performance requirements must be met
to ensure that the CI can detect improper sterilization
conditions inside of each pack/container. Figure 1
shows three typical Stated Values for the 3M™ Attest™
Steam Chemical Integrators.
Instructions For Use
Placement and Processing
1. Carefully tear or use scissors at the tear notch at the
top of the foil package to make the initial opening.
Remove only the number of 3M™ Attest™ Steam
Chemical Integrators needed. Reseal the package.
2. Place a 3M™ Attest™ Steam Chemical Integrator in
each pack, peel pouch, container system or tray to
be steam sterilized in the area determined to be the
least accessible to steam penetration.
3. Process the load according to established
procedures.
Note: Refer to the package insert for a complete set
of instructions.
After processing, the dark color should have entered
the ACCEPT window of the 3M™ Attest™ Steam
Chemical Integrators 1243A, 1243B and 1243RE. If the
dark color has not entered the ACCEPT window, this
indicates a REJECT result which means that the items
in the pack, peel pouch, container system, or tray were
not exposed to sucient steam sterilization conditions.
These items should be returned for reprocessing.
Page 7

3M™ Attest™ Steam Chemical Integrator Technical Information
Interpretation of Results
Unprocessed
Processed — ACCEPT Processed — REJECT
7
If the color bar reaches or crosses into the ACCEPT
window, it is a pass and the necessary conditions
of time, temperature and steam have been met for
sterilization.
ACCEPT
If the color bar is in the REJECT region or on the line, it
is considered a fail. The pack should be reprocessed and
the cause of sterilization failure should be investigated.
REJECT
Page 8

Safety
The design of the 3M™ Attest™ Steam Chemical Integrator prevents the indicating chemicals of the CI from coming in
contact with sterilized materials or personnel handling the device. The chemical, as a pellet before processing or as a
melted color front after processing, is contained in an envelope of impermeable top and bottom layers.
Storage and Shelf Life
• Best stored at normal room temperature conditions, 15–30ºC (59–86ºF) and 40–60% relative humidity. Store
away from direct sunlight. Do not store near strong alkaline or acidic products such as cleaning/disinfecting agents.
• After use, the indicator will not change visually within 6 months when stored at above conditions.
• 3M™ Attest™ Steam Chemical Integrators contained in an unopened package have a shelf life as labeled from date
of manufacture when stored at recommended conditions. The expiration date is printed on the package label and
is also contained in the barcode on each device.
References
1
Perkins, J.J., Principles and Methods of Sterilization In Health Sciences, ed 2, Springeld, IL,
Charles C. Thomas, 1976.
2
Validation of Moist Heat Sterilization Processes: Cycle Design, Development, Qualication and Ongoing
Control, Technical Report No. 1 (Revised 2007), PDA Journal of Pharmaceutical Science and Technology,
Supplement Vol. 61, No. S-1, 2007.
3
International Standard. Sterilization of health care products Biological indicators — Part 7: Guidance
for the selection, use and interpretation of results, ANSI/AAMI/ISO 11138-7:2019.
4
Association for the Advancement of Medical Instrumentation. Comprehensive guide to steam sterilization
and sterility assurance in health care facilities, ANSI/AAMI ST79: 2017.
5
The Association of periOperative Registered Nurses (AORN) Recommended Practices for Sterilization in
Perioperative Practice Settings, 2020.
6
The Association of periOperative Registered Nurses (AORN) Recommended Practices for Selection and Use
of Packaging Systems for Sterilization, 2020.
7
International Standard. Sterilization of health care products-Chemical indicators-Part 1: General requirements,
ISO 11140-1:2014.
3M Medical Solutions Division
3M Health Care
3M Center, Building 275-4E-01
St. Paul, MN 55144 USA
Phone 1-800-228-3957
Web 3M.com/Medical
Learn more on our website:
3M.com/Attest
© 3M 2020. All rights reserved. 3M and Attest are marks and/or registered marks of 3M.
Used under license by 3M subsidiaries and aliates. Unauthorized use prohibited. Please recycle.
Printed in USA. 70-2009-0710-6_US
 Loading...
Loading...