Welch Allyn ProBP 2000 Directions For Use Manual

Welch Allyn ProBP™ 2000 Digital
Blood Pressure Device
Directions for use
Software Version 1.X

© 2018 Welch Allyn. All rights are reserved. To support the intended use of the product described in this
publication. The purchaser of the product is permitted to copy this publication, for internal distribution
only, from the media provided by Welch Allyn. No other use, reproduction, or distribution of this
publication, or any part of it, is permitted without written permission from Welch Allyn. Welch Allyn
assumes no responsibility for any injury to anyone, or for any illegal or improper use of the product, that
may result from failure to use this product in accordance with the instructions, cautions, warnings, or
statement of intended use published in this manual.
SureBP® technology and Welch Allyn FlexiPort® are registered trademarks of Welch Allyn.
The Bluetooth® word mark and logos are registered trademarks owned by BluetoothSIG, Inc. and any
use of such marks by Welch Allyn is under license.
Software in this product is © 2018 Welch Allyn or its vendors. All rights are reserved. The software is
protected by United States of America copyright laws and international treaty provisions applicable
worldwide. Under such laws, the licensee is entitled to use the copy of the software incorporated with
this instrument as intended in the operation of the product in which it is embedded. The software may
not be copied, decompiled, reverse engineered, disassembled, or otherwise reduced to humanperceivable form. This is not a sale of the software or any copy of the software; all right, title, and
ownership of the software remain with Welch Allyn or its vendors.
For information about any Welch Allyn product, contact Welch Allyn Technical Support:
www.welchallyn.com/support.
DIR 80021232 Ver. E
Revised: 2018-10
Distributed by Welch Allyn, Inc.
4341 State Street Road
Skaneateles Falls, NY 13153 USA
www.welchallyn.com
Guangdong Transtek Medical Electronics Co., Ltd.
No. 105 Dongli Road
Torch Development District
Zhongshan, 528437, Guangdong, China
Made in China
This manual applies to the 901123 Digital Blood Pressure Device
Authorized Representative in the European Community
MDSS -Medical Device Safety Service GmbH
Schiffgraben 41, 30175
Hannover, Germany
http://

Contents
Introduction ............................................................................................. 1
iii
Intended use/Indications for use .......................................................................... 1
Contraindications ................................................................................................. 1
Symbols ............................................................................................................... 2
About warnings and cautions ............................................................................... 5
Contents list ......................................................................................................... 7
Controls and indicators ........................................................................................ 8
Power options ...................................................................................................... 9
Screen elements ................................................................................................ 10
Insert or replace the batteries ............................................................................ 11
Position the blood pressure cuff on the patient ................................................. 12
Maintenance .......................................................................................... 13
Maintain the device ............................................................................................ 13
Troubleshooting ................................................................................................ 14
Specifications ........................................................................................ 17
Transducer accuracy test ................................................................................... 19
Complied standards list ...................................................................................... 21
General radio compliance .................................................................... 23
Federal Communication Commission (FCC) Interference Statement ................ 23
FCC Radiation Exposure Statement ................................................................... 23
Industry Canada (IC) compliance ........................................................................ 24
European Union ................................................................................................. 24
Warranty ................................................................................................ 27
Approved accessories ........................................................................... 29
EMC guidance and manufacturer’s declarations ................................ 31
EMC guidance .................................................................................................... 31
Emissions and immunity information ................................................................. 32

iv Contents Welch Allyn ProBP™ 2000 Digital Blood Pressure Device
Introduction
Readings taken by the device are equivalent to those obtained by a trained observer
using the cuff and stethoscope auscultation method.
This Directions for use contains important safety and care information and provides stepby-step instructions for using the device. Read the manual thoroughly before using the
device.
1
Intended use/Indications for use
The Welch Allyn ProBP 2000 Digital blood pressure device is intended for use in
measuring blood pressure and heart rate in pediatric and adult patient populations 3+
years with arm circumferences between 15 cm to 55 cm (approximately 5.9 to 21.7
inches).
The Welch Allyn ProBP 2000 automatically measures systolic and diastolic pressure and
pulse rate, as well as calculates Mean Arterial Pressure (MAP). The device is intended to
be used by clinicians and medically qualified personnel.
Contraindications
This device is not intended for use on neonates, infants, or children under the age of 3
years. The effectiveness of this device has not been established in pregnant, including
pre-eclamptic, patients.

2 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device
Symbols
Documentation symbols
Warning: The warning statements in this manual identify conditions or practices that could lead to
illness, injury, or death.
Caution: The caution statements in this manual identify conditions or practices that could result in
damage to the equipment or other property, or loss of data.
Follow instructions/directions for use (DFU) -- mandatory action. A copy of the DFU is available on this
website. A printed copy of the DFU can be ordered from Welch Allyn for delivery within 7 calendar days.
Power symbols
Direct current
Power
Shipping, storing, and environment symbols
Humidity limitation
Separate collection of Electrical and Electronic Equipment. Do not dispose as unsorted
municipal waste.
Temperature limit
Atmospheric pressure limitation
Recyclable
Directions for use Introduction 3
Stacking limit by number
Cuff symbols
Artery marker
Range
Artery index marker
Miscellaneous symbols
Limb circumference (Minimum/Maximum)
Lot Code
Not made with natural rubber latex
Authorized Representative in the European Community
Manufacturer
Date of manufacture
Type BF applied part
Serial Number
4 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device
Product Identifier
Lot Code
Reorder Number
Non-ionizing electromagnetic radiation
Australian Communications and Media Authority (ACMA) Radio Compliance
Mark (RCM)
Global Trade Item Number
IP22
Class II equipment
Ingress protection: the device is protected against solid foreign objects of 12.
5mm and greater and against vertically falling water drops when
ENCLOSURE is tilted up to 15°
Prescription only or "For Use by or on the order of a licensed medical
professional"
Meets essential requirements of the European Medical Device Directive 93/
42/EC
The product contains certain hazardous substances.
Bluetooth
Note Your model might not contain all of these features.
Directions for use Introduction 5
About warnings and cautions
Warning and caution statements can appear on the Welch Allyn ProBP™ 2000 Digital
Blood Pressure Device, the packaging, the shipping container, or in this Directions for
use.
Warnings and cautions
WARNING Patient injury risk. The device is not suitable for measuring the
blood pressure of neonatal infants or children.
WARNING Patient injury risk. The decision to use the device on pregnant
or pre-eclamptic patients is at the discretion of the trained clinician using
the equipment.
WARNING Injury risk. Do not burn batteries. Batteries may leak or
explode.
WARNING Patient injury risk. If the patient experiences discomfort during
a measurement, such as pain in the arm or other complaints, press the
Power button immediately to release the air from the cuff. Loosen and
remove the cuff from the patient's arm.
WARNING Patient injury risk. On the rare occasion of a fault causing the
cuff to remain fully inflated during measurement, open the cuff
immediately. Prolonged high pressure applied to the arm (cuff pressure
>300mmHg or constant pressure >15mmHg for more than 3 minutes)
might lead to bruising and discolored skin.
WARNING Patient injury risk. This unit is not suitable for continuous
monitoring during medical emergencies or operations.
WARNING Patient injury risk. Taking blood pressure measurements too
frequently could disrupt blood circulation and cause injuries.
WARNING Patient injury risk. Do not place the cuff on the arm on the
same side of a mastectomy. If necessary, use the femoral artery in the
thigh to take a measurement.
WARNING Patient injury risk. Do not kink the connection tube during use.
The cuff pressure might continuously increase, which could prevent blood
flow and result in injury.
WARNING Patient injury risk. Do not apply cuff to areas on patient where
skin is delicate or damaged. Check cuff site frequently for irritation.
WARNING Patient injury risk. Do not use the unit if the patient is allergic
to polyester or synthetic materials.
WARNING Patient injury risk. Do not connect the air tube to other medical
equipment. This could cause air to be pumped into intravascular systems or
high pressure, which could lead to serious injuries.
WARNING Patient injury risk. The device has not been designed for use
with high-frequency (HF) surgical equipment and does not protect against
hazards to the patient.

6 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device
WARNING Inaccurate measurement risk. Do not place the cuff where it
can disturb proper circulation. Do not place the cuff on any area where
circulation is compromised or on any extremity used for intravenous
infusions. Do not use an SpO2 finger clip sensor and a blood pressure cuff
simultaneously on the same limb. Doing so may cause a temporary loss of
pulsatile flow, resulting in either no reading or an inaccurate SpO2 or pulse
rate until the flow returns.
WARNING Inaccurate measurement risk. Do not use the device on
patients who are on heart-lung machines.
WARNING Inaccurate measurement risk. Do not use the device on
patients who are experiencing convulsions or tremors.
WARNING Injury risk. Do not touch output of the batteries/adapter and the
user simultaneously.
WARNING Injury risk. Excessive tube lengths could cause strangulation if
you don't manage them properly.
WARNING Injury risk. Dispose of accessories, detachable parts, and the
device according to the local guidelines.
WARNING Injury risk. Do not service or perform any maintenance while
using the device.
WARNING Injury risk. Use only accessories approved by the
manufacturer. Using unapproved accessories might cause damage to the
unit and injure users.
WARNING Injury risk. No modification to this equipment is allowed.
Modifying the equipment could damage the unit or endanger the user.
WARNING The power cord is considered the disconnect device for
isolating this equipment from supply mains. Do not position the equipment
so that it is difficult to reach or disconnect.
CAUTION This device is intended for non-invasive measuring and
monitoring of arterial blood pressure. It is not intended for use on
extremities other than the arm or for purposes other than obtaining a blood
pressure measurement.
CAUTION United States Federal law restricts this device to sale,
distribution, or use by or on the order of a physician or licensed healthcare
professional.
CAUTION Do not wrap the cuff on the same arm to which another
monitoring device is applied. One or both devices could temporarily stop
functioning if you try to use them on the same arm at the same time.
CAUTION To avoid measurement errors, avoid taking blood pressure
measurements near a strong electromagnetic field radiated interference
signal or electrical fast transient/burst signal.
CAUTION Use the device in the environment described in this directions
for use. Otherwise, you will compromise the device's performance and
reduce its lifetime.

Directions for use Introduction 7
CAUTION Do not attempt to repair the unit yourself if it malfunctions.
Only have repairs carried out by authorized service centers.
CAUTION Report any unexpected operation or events to the
manufacturer.
CAUTION Use a soft cloth to clean the entire unit. Do not use any
abrasive or volatile cleaners. See the cleaning instructions presented later
in this Directions for use.
Contents list
The following items are in the box:
• Blood pressure device
• REUSE-11 Adult cuff (25–34cm)
• (4) AA alkaline batteries
8 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device
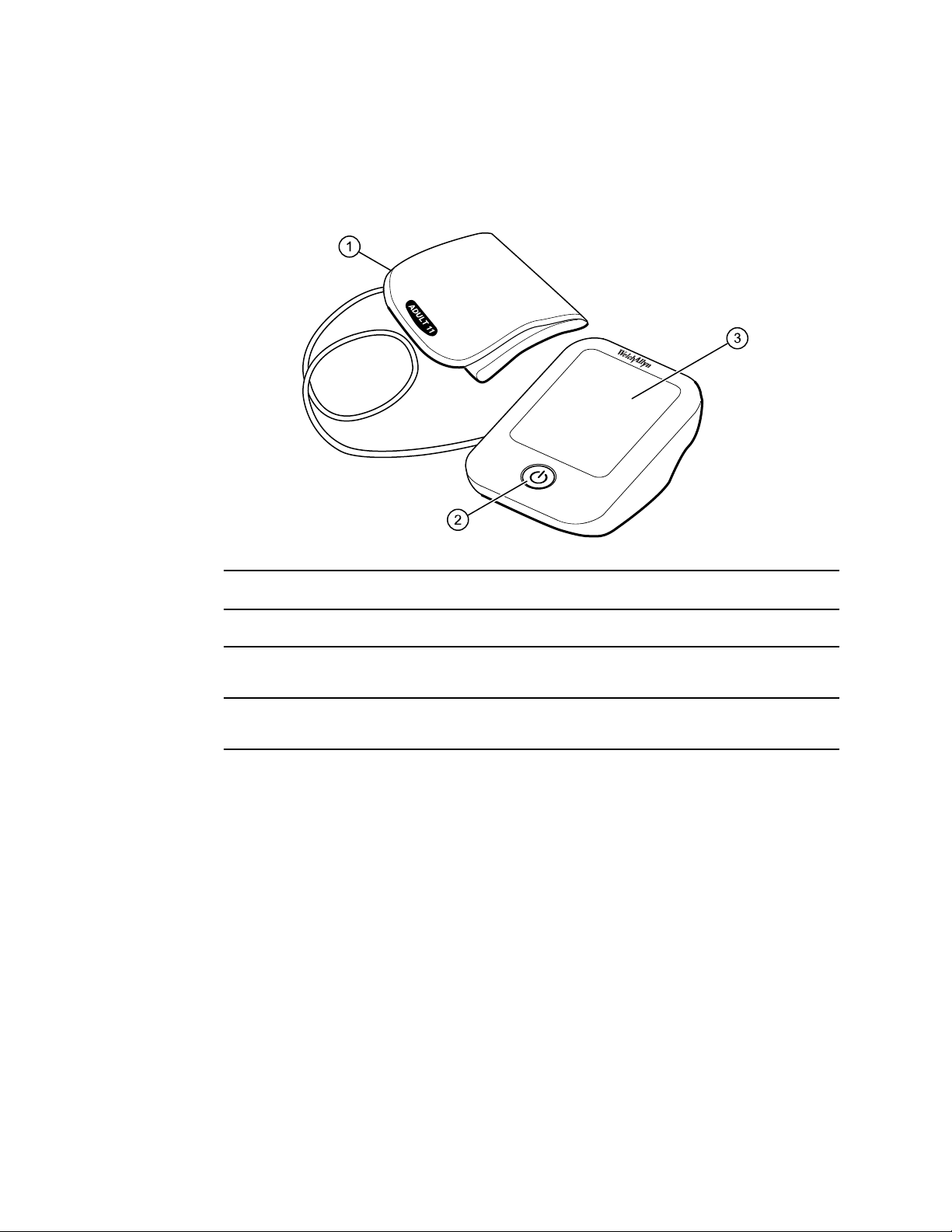
Controls and indicators
Device front
No. Feature Description
1
FlexiPort® blood pressure cuff
2 Power button Powers on the blood pressure device and starts and stops a blood
3 LCD Display Displays blood pressure reading and other pertinent information
Apply to upper arm to take a blood pressure measurement
pressure measurement
regarding the reading
 Loading...
Loading...