Welch Allyn Connex IWS Directions For Use Manual
Welch Allyn Connex
®
Devices
Directions for use
Software version 2.3X
© 2017 Welch Allyn. All rights are reserved. To support the intended use of the product described in this publication, the
purchaser of the product is permitted to copy this publication, for internal distribution only, from the media provided by
Welch Allyn. No other use, reproduction, or distribution of this publication, or any part of it, is permitted without written
permission from Welch Allyn. Welch Allyn assumes no responsibility for any injury to anyone, or for any illegal or improper
use of the product, that may result from failure to use this product in accordance with the instructions, cautions, warnings,
or statement of intended use published in this manual.
Welch Allyn, Connex, SureTemp, FlexiPort, and SureBP are registered trademarks of Welch Allyn.
Vital Signs Monitor 6000 Series and PartnerConnect are trademarks of Welch Allyn.
Integrated Pulmonary Index is a trademark of , and Oridion and Microstream are registered trademarks of, Oridion Medical
1987 Ltd. No implied license. Possession or purchase of this device does not convey any express or implied license to use
the device with unauthorized CO2 sampling products which would, alone, or in combination with this device, fall within the
scope of one or more of the patents relating to this device and/or CO2 sampling products.
Radical-7R, Pulse CO-Oximeter, rainbow Acoustic Monitoring, RRa, and ReSposable are trademarks of, and SET, LNCS,
SpHb, rainbow, and Masimo are registered trademarks of, Masimo Corporation. Possession or purchase of a Masimoequipped device does not convey any express or implied license to use the device with unauthorized sensors or cables
which would, alone or in combination with this device, fall within the scope of one or more of the patents relating to this
device.
Nellcor SpO2 Patient Monitoring System with OxiMax Technology and Nellcor SpO2 OxiMax Technology are registered
trademarks of COVIDIEN LP Covidien Holdings Inc.
Braun and ThermoScan are registered trademarks of Braun GmbH.
Health o meter is a registered trademark of Sunbeam Products, Inc., used under license.
EarlySense is a registered trademark of EarlySense Ltd.
Software in this product is Copyright 2017 Welch Allyn or its vendors. All rights are reserved. The software is protected by
United States of America copyright laws and international treaty provisions applicable worldwide. Under such laws, the
licensee is entitled to use the copy of the software incorporated with this instrument as intended in the operation of the
product in which it is embedded. The software may not be copied, decompiled, reverse-engineered, disassembled, or
otherwise reduced to human-perceivable form. This is not a sale of the software or any copy of the software; all right, title,
and ownership of the software remain with Welch Allyn or its vendors.
For patent information, please visit
www.welchallyn.com/patents.
For Nellcor patent information, please visit www.covidien.com/patents.
For information about any Welch Allyn product, contact Welch Allyn Technical Support: www.welchallyn.com/about/
company/locations.htm.
DIR 80021750 Ver. C
Revision date: 2017-09
Welch Allyn, Inc.
4341 State Street Road
Skaneateles Falls, NY 13153-0220 USA
www.welchallyn.com
This manual applies to 901060 Vital Signs Monitor and 901028
Connex Integrated Wall System.
Regulatory Affairs Representative
Welch Allyn Limited
Navan Business Park
Dublin Road
Navan, County Meath
Republic of Ireland

Contents
Introduction ............................................................................................. 1
Symbols ................................................................................................... 5
Screen elements ...................................................................................... 9
iii
Connex Vital Signs Monitor 6000 Series .............................................................. 1
Connex Integrated Wall System .......................................................................... 1
Indications for use ................................................................................................ 2
Contraindications ................................................................................................. 3
About warnings and cautions .............................................................. 15
General warnings and cautions .......................................................................... 15
Physical design ...................................................................................... 23
Vital Signs Monitor 6000 Series ......................................................................... 23
Integrated Wall System ..................................................................................... 27
Setup ...................................................................................................... 31
Vital Signs Monitor 6000 Series ......................................................................... 31
Supplies and accessories ................................................................................... 31
Insert the battery ............................................................................................... 31
Mount the monitor ............................................................................................. 32
Attach the probe well ......................................................................................... 33
Attach the temperature probe ........................................................................... 34
Remove the temperature probe and well .......................................................... 34
Connect the NIBP hose ..................................................................................... 35
Disconnect the NIBP hose ................................................................................. 35
Connect the SpO2 cable or the SpO2/RRa dual cable ....................................... 35
Disconnect the SpO2 cable or the SpO2/RRa dual cable .................................. 37
Connect the patient movement cable ................................................................ 37
Disconnect the patient movement sensor and cable ........................................ 38
Attach a USB accessory ..................................................................................... 38
Detach a USB accessory .................................................................................... 39
Insert a new roll of paper ................................................................................... 39
Connect AC power ............................................................................................. 40
Disconnect AC power ........................................................................................ 40
Integrated Wall System ..................................................................................... 41
Supplies and accessories ................................................................................... 41
Unpack the wall system ..................................................................................... 41
Insert the battery ............................................................................................... 42
Prepare for mounting ......................................................................................... 43
Mounting location .............................................................................................. 44
Mount the wall system ...................................................................................... 44

iv Contents Welch Allyn Connex® Devices
Mount the accessory bin ................................................................................... 48
Connect the blood pressure (NIBP) hose ........................................................... 50
Set up the physical assessment instrument handles and specula dispenser ... 50
Set up the SureTemp® Plus thermometer ........................................................ 51
Set up the Braun ThermoScan® PRO thermometer ......................................... 52
Connect AC power ............................................................................................. 52
Attach an accessory ........................................................................................... 53
Startup .................................................................................................... 55
Power ................................................................................................................. 55
Power up the monitor ........................................................................................ 56
Set the date and time ........................................................................................ 57
Power down the monitor ................................................................................... 57
Reset the monitor .............................................................................................. 58
Enter clinician information .................................................................................. 58
Navigation .............................................................................................. 59
Home tab ........................................................................................................... 59
Device Status area ............................................................................................. 59
Content area ...................................................................................................... 62
Navigation area .................................................................................................. 64
Using the keypad, keyboard, and barcode scanner ........................... 67
Open the numeric keypad .................................................................................. 67
Numeric keypad ................................................................................................. 67
Enter a number .................................................................................................. 68
Close the numeric keypad ................................................................................. 68
Open the keyboard ............................................................................................ 68
Keyboard ............................................................................................................ 68
Enter a letter or number ..................................................................................... 70
Enter a symbol or special character ................................................................... 70
Enter a diacritical mark ....................................................................................... 70
Close the keyboard ............................................................................................ 71
Use a barcode scanner ...................................................................................... 71
Host system ........................................................................................... 73
Overview ............................................................................................................ 73
Monitor tab ........................................................................................................ 74
Connect to the central station ........................................................................... 75
Disconnect from the central station .................................................................. 75
Continuous patient monitoring ........................................................................... 75
Activate the Continuous Monitoring profile ...................................................... 76
Pause continuous monitoring (Pause mode) ..................................................... 77
Resume continuous monitoring ........................................................................ 78
End continuous monitoring ............................................................................... 78
Assign a patient and location ............................................................................. 79
Profiles ................................................................................................... 81
Continuous Monitoring profile ........................................................................... 81
Saving vital sign measurements (Continuous Monitoring profile) ...................... 83
Intervals Monitoring profile ................................................................................ 83
Spot Check profile .............................................................................................. 84
Office profile ...................................................................................................... 84

Directions for use Contents v
Profile feature comparison ................................................................................. 85
Changing profiles ............................................................................................... 86
Patient data management .................................................................... 91
Add a patient to the patient list .......................................................................... 91
Load patient data with a barcode scanner ......................................................... 92
Select a patient .................................................................................................. 92
Office profile ...................................................................................................... 94
Manage patient records (Continuous Monitoring profile) .................................. 95
Manage patient records (Intervals Monitoring, Spot Check, and Office
profiles) .............................................................................................................. 97
Printer ................................................................................................................ 98
Delete a patient from the list ............................................................................. 98
Alarms .................................................................................................. 101
Patient rest mode ............................................................................................ 105
Reset (pause or turn off) audio alarms ............................................................. 106
Cancel a paused alarm ..................................................................................... 107
Adjust vital sign alarm limits ............................................................................ 107
Modify audio alarm notification ........................................................................ 108
Alarm messages and priorities ......................................................................... 109
Nurse call ......................................................................................................... 113
Patient monitoring .............................................................................. 115
Standard and custom modifiers ....................................................................... 115
Custom scoring ................................................................................................ 116
Manual overrides ............................................................................................. 116
Patient movement ........................................................................................... 116
Capnography (CO2) .......................................................................................... 122
Respiration rate ................................................................................................ 126
IPI ..................................................................................................................... 128
Acoustic respiration rate (RRa) ......................................................................... 131
NIBP ................................................................................................................. 134
Temperature .................................................................................................... 150
SpO2 ................................................................................................................ 163
SpHb ................................................................................................................ 169
Pulse rate frame ............................................................................................... 173
Manual parameters frame ................................................................................ 175
Physical assessment instrument handles ........................................................ 176
Maintenance and service .................................................................... 179
Perform periodic checks .................................................................................. 179
Replace the printer paper (Connex VSM) ......................................................... 180
Change the battery (Connex VSM) .................................................................. 181
Remove the wall system from the wall (Connex IWS) .................................... 182
Change the battery (Connex IWS) ................................................................... 183
Clean the monitor ............................................................................................ 184
Prepare to clean the equipment ....................................................................... 185
Clean the accessories ...................................................................................... 186
Advanced settings ............................................................................... 187
General ............................................................................................................. 187
Parameters ....................................................................................................... 192

vi Contents Welch Allyn Connex® Devices
Data management ........................................................................................... 199
Network ........................................................................................................... 202
Service ............................................................................................................. 205
Troubleshooting .................................................................................. 207
Patient movement messages .......................................................................... 207
CO2 messages ................................................................................................ 209
RRa messages ................................................................................................. 210
NIBP messages ............................................................................................... 211
SpO2 and SpHb messages .............................................................................. 212
Temperature messages ................................................................................... 213
Weight scale messages ................................................................................... 214
Physical assessment instrument handles ........................................................ 214
Patient data management messages .............................................................. 215
Communications module messages ................................................................ 216
Radio messages ............................................................................................... 216
Ethernet messages .......................................................................................... 216
USB and USB flash drive messages ................................................................ 216
System messages ........................................................................................... 217
Battery power manager messages .................................................................. 217
Configuration Manager messages ................................................................... 218
Printer messages ............................................................................................. 218
Network messages .......................................................................................... 219
Problems and solutions .................................................................................... 219
Specifications ...................................................................................... 221
Physical specifications ..................................................................................... 221
Environmental specifications ........................................................................... 236
Device radio ..................................................................................................... 236
Configuration options ....................................................................................... 236
Vital Signs Monitor 6000 Series ....................................................................... 236
Integrated Wall System ................................................................................... 237
Standards and compliance ................................................................. 239
General compliance and standards .................................................................. 239
General radio compliance ................................................................................. 240
Guidance and manufacturer's declaration ........................................ 245
EMC compliance .............................................................................................. 245
Emissions and immunity information ............................................................... 245
Appendix .............................................................................................. 247
Approved accessories ...................................................................................... 247
Warranty .......................................................................................................... 258
Introduction
This directions for use (DFU) covers the following devices:
• the Connex® Vital Signs Monitor 6000 Series (Connex VSM or VSM 6000 series)
• the Connex® Integrated Wall System (Connex IWS)
Most of the content in this directions for use applies to both Connex devices, but some
sections present device-specific content. See section titles and parentheses throughout
to identify the device-specific content.
1
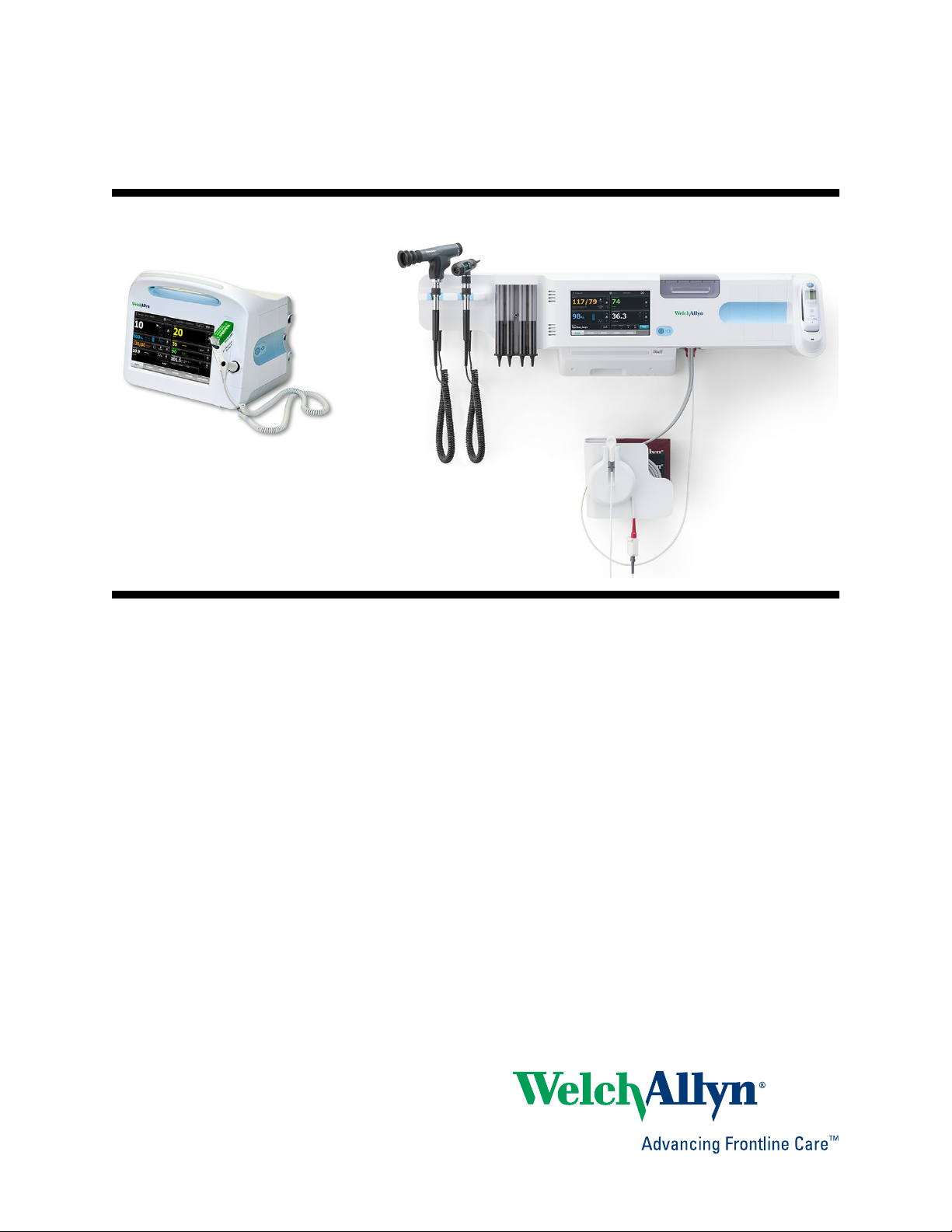
Connex Vital Signs Monitor 6000 Series
This directions for use describes the capabilities and operation of the monitor. The
information, including the illustrations, covers all configuration options. If your monitor
configuration lacks any of these options, some information in this manual might not
apply.
Before using the monitor, you must familiarize yourself with all warnings and cautions,
with the steps to power up the monitor, and with the sections of this directions for use
that pertain to your use of the monitor. You must also familiarize yourself with all
information that accompanies the accessories you use.
Note Some product features described in this publication might not be available
in your country. For the latest information about products and features,
please call Welch Allyn Customer Care.
Connex Integrated Wall System
The Welch Allyn Connex Integrated Wall System combines the advanced, easy-to-use
monitor capabilities of the Welch Allyn Connex Vital Signs Monitor 6000 Series with the
Welch Allyn 767 Power Handles. This manual (directions for use) is designed to help you
understand the capabilities and operation of the wall system. The information in this
manual, including the illustrations, is based on a wall system configured with noninvasive blood pressure (NIBP), body temperature, pulse oximetry (SpO2), total
hemoglobin concentration (SpHb), pulse rate, weight scale, and two power handles. If
your wall system configuration lacks any of these options, some information in this
manual may not apply.
Before using the wall system, read the sections of the manual that pertain to your use of
the system.
Note
Throughout this directions for use, the Integrated Wall System may be
referred to as a wall system or monitor.

2
Introduction Welch Allyn Connex® Devices
Note Some product features described in this publication might not be available
in your country. For the latest information about products and features,
please call Welch Allyn Customer Care.
Indications for use
The Connex VSM 6000 series of monitors is intended to be used by clinicians and
medically qualified personnel for monitoring of neonatal, pediatric, and adult patients for
• noninvasive blood pressure (NIBP)
• pulse rate (PR)
• noninvasive functional oxygen saturation of arteriolar hemoglobin (SpO2)
• body temperature in normal and axillary modes
The most likely locations for patients to be monitored are general medical and surgical
floors, general hospital, and alternate care environments. Monitoring can be
accomplished on the VSM 6000 series bedside monitor itself, and the VSM 6000 series
bedside monitor also can transmit data continuously for secondary remote viewing and
alarming (e.g., at a central station). Secondary remote viewing and alarming features are
intended to supplement and not replace any patient bedside monitoring procedures.
The optional Masimo rainbow® SET Pulse CO-Oximeter™ and accessories are indicated
for the continuous noninvasive monitoring of functional oxygen saturation of arterial
hemoglobin (SpO2), pulse rate (PR), total hemoglobin concentration (SpHb®), and/or
respiration rate (RRa™). The Masimo rainbow SET Radical-7R™ Pulse CO-Oximeter and
accessories are indicated for use with adult, pediatric, and neonatal patients during both
motion and no-motion conditions, and for patients who are well or poorly perfused in
hospitals and hospital-type facilities.
The optional Oridion®module and accessories are intended for the continuous
noninvasive measurement and monitoring of carbon dioxide concentration of the expired
and inspired breath (etCO2 and FiCO2) and respiration rate (RR). It is intended for use
with neonatal, pediatric, and adult patients in hospitals and hospital-type facilities.
The optional Oridion module also provides the clinician with an Integrated Pulmonary
Index™ (IPI). The IPI is based on four parameters provided by the monitor: end-tidal
carbon dioxide (etCO2), respiration rate (RR), oxygen saturation (SpO2), and pulse rate
(PR). The IPI is a single index of an adult or pediatric patient's ventilatory status displayed
on a scale of 1–10, where 10 indicates optimal pulmonary status. IPI monitoring displays
a single value that represents the patient's pulmonary parameters and alerts clinicians to
changes in the patient's pulmonary status.
The IPI is an adjunct to, and is not intended to replace, vital signs monitoring.
Optional compatible weight scales (e.g., Health o meter®) can be used for height,
weight, and BMI input.
The optional EarlySense® (Everon) System is intended for continuous measurement of
respiration rate, heart rate, and movement in an automatic contact-less manner, in a
hospital or clinic setting. The system is indicated for use in children, adolescents, and
adults. The operation of the EarlySense has been studied in children (weight ≥ 10 Kg)
and adults (weight <111 Kg) during sleep and resting condition.
This product is available for sale only upon the order of a physician or licensed healthcare
professional.

Directions for use Introduction 3
Handle module assembly (Integrated Wall System only)
Handles supply power to Welch Allyn 3.5V instruments.
Contraindications
This system (all configurations) is not intended to be used:
• on patients connected to heart/lung machines
• on patients being transported outside a healthcare facility
• within the controlled access area of MRI equipment
• in a hyperbaric chamber
• in the presence of flammable anesthetics
For contraindications of SpO2 and SpHb sensors, consult the sensor manufacturer's
directions for use.
Systems configured with EarlySense are not intended to be used:
• on patients for whom proper positioning cannot be achieved or maintained
• on patients who do not meet the weight limits tested or specified

4 Introduction Welch Allyn Connex® Devices
Symbols
For information on the origin of these symbols, see the Welch Allyn symbols glossary:
www.welchallyn.com/symbolsglossary.
Documentation symbols
WARNING The warning statements in this manual identify conditions or practices that could
lead to illness, injury, or death.
5
Power symbols
CAUTION The caution statements in this manual identify conditions or practices that could
result in damage to the equipment or other property, or loss of data. This definition applies to
both yellow and black and white symbols.
WARNING Hot surface. Do not touch.
Follow the operating instructions/directions for use (DFU) — mandatory action.
A copy of the DFU is available on this website.
A printed copy of the DFU can be ordered from Welch Allyn for delivery within 7 days.
Power on/Display power-saving
[recent models]
Power on/Display power-saving
[older models]
Equipotential terminal
(on the display) monitor is plugged
into Alternating Current power
Battery absent or faulty
6 Symbols Welch Allyn Connex® Devices
(on the monitor, green indicator)
Alternating Current power present,
battery fully charged
(on the monitor, amber indicator)
Alternating Current power present,
battery is charging
Alternating Current (AC) Rechargeable battery
Li-ion battery AC input power
Connectivity symbols
USB
Wireless signal strength
• Best (4 bars)
• Good (3 bars)
• Fair (2 bars)
• Weak (1 bar)
• No signal (no bars)
• No connection (blank)
Battery charge level
Battery cover
Ethernet RJ-45
Nurse call
[recent models]
Nurse call
[older models]
Connected to central station Disconnected from central station
Miscellaneous symbols
CO2 sampling input
Manufacturer Limited rotation/Turn completely to
Reorder number Serial number
Do not reuse China RoHS markings for control of
CO2 sampling output/exhaust
right
pollution caused by electronic
information products. XX indicates
Environmentally Friendly Use
Period in years.
Directions for use Symbols 7
Nonionizing electromagnetic
radiation
Restrictions for use of wireless
device in Europe. European
Community's Class 2 radio
equipment.
Defibrillation-proof Type BF applied
parts
Atmospheric pressure limitation Not for injection
Transport and storage temperature
range
Maximum safe working load limits
(specific values presented with
symbol)
Recycle the product separate from
other disposables
Call for maintenance
Defibrillation-proof Type CF applied
parts
Authorized Representative of the
European Community
Mass in kilograms (kg)
Recycle
Do not expose to open flame
IPX1
(Vital Signs
Monitor)
IPX0
(Integrated Wall
System)
Degree of protection provided by
the enclosure with respect to
harmful ingress of liquids

8 Symbols Welch Allyn Connex® Devices
Screen elements
Global navigation, controls, and indicators
Select option Process indicator for
9
activities like acquiring
measurements and
connecting to a central
station
Select item from list Display lock/unlock
Increase or decrease value
Monitoring and connectivity
Connect to the central station
and retain patient data
(Monitor tab)
Connected to the central
station (Device Status area)
Temporarily pause continuous
monitoring but retain patient
data
Disconnect from the central
station but continue
monitoring and retain patient
data (Monitor tab)
Disconnected from the
central station (Device Status
area)
End continuous monitoring
session for current patient
and clear patient data

10 Screen elements Welch Allyn Connex® Devices
NIBP
NIBP start NIBP stop
Intervals status indicators NIBP view toggle
Temperature
Temperature site control Direct mode selector
SpO2 and Pulse rate
Pulse amplitude bar SatSeconds timer (Nellcor
SpO2 view toggle Response mode selector (Fast
Beats per minute (to
represent pulse rate)
Total hemoglobin (SpHb)
SpHb view toggle Averaging mode selector
Capnography (CO2)
feature only)
mode selected)
(Long mode selected)
CO2 pump start CO2 pump stop

Directions for use Screen elements 11
Capnography (CO2)
etCO2 view toggle IPI view toggle
IPI graphic indicators
RRa
Respiration indicator Averaging mode selector
(Fast mode selected)
Patient movement
Manual parameters
Bed mode Bed exit
Patient turn indicator and
timer
Patient turn indicator (Review
tab)
Bed exit (Review tab)
Exit sensitivity indicator
Manual parameter selector
12 Screen elements Welch Allyn Connex® Devices
Alarm and information messages
Alarm limit control
Multiple alarms toggle Alarm audio paused
Alarm active Information message
Patient Rest Mode
Patient data management
Diacritical marks key
(available for languages that
use diacritical marks;
appearance differs based on
language)
Alarm On/Off toggle
Symbols key
Send patient data Print patient data
View tabular trend data View graphical trend data
Cancel action Add patient identifiers
Retrieve patient list from the
network
Delete patient from List tab Clear patient context from
Forward or backward in
Review tab
Select patient from List tab
Summary tab
Proceed to the next field to
input patient information
Directions for use Screen elements 13
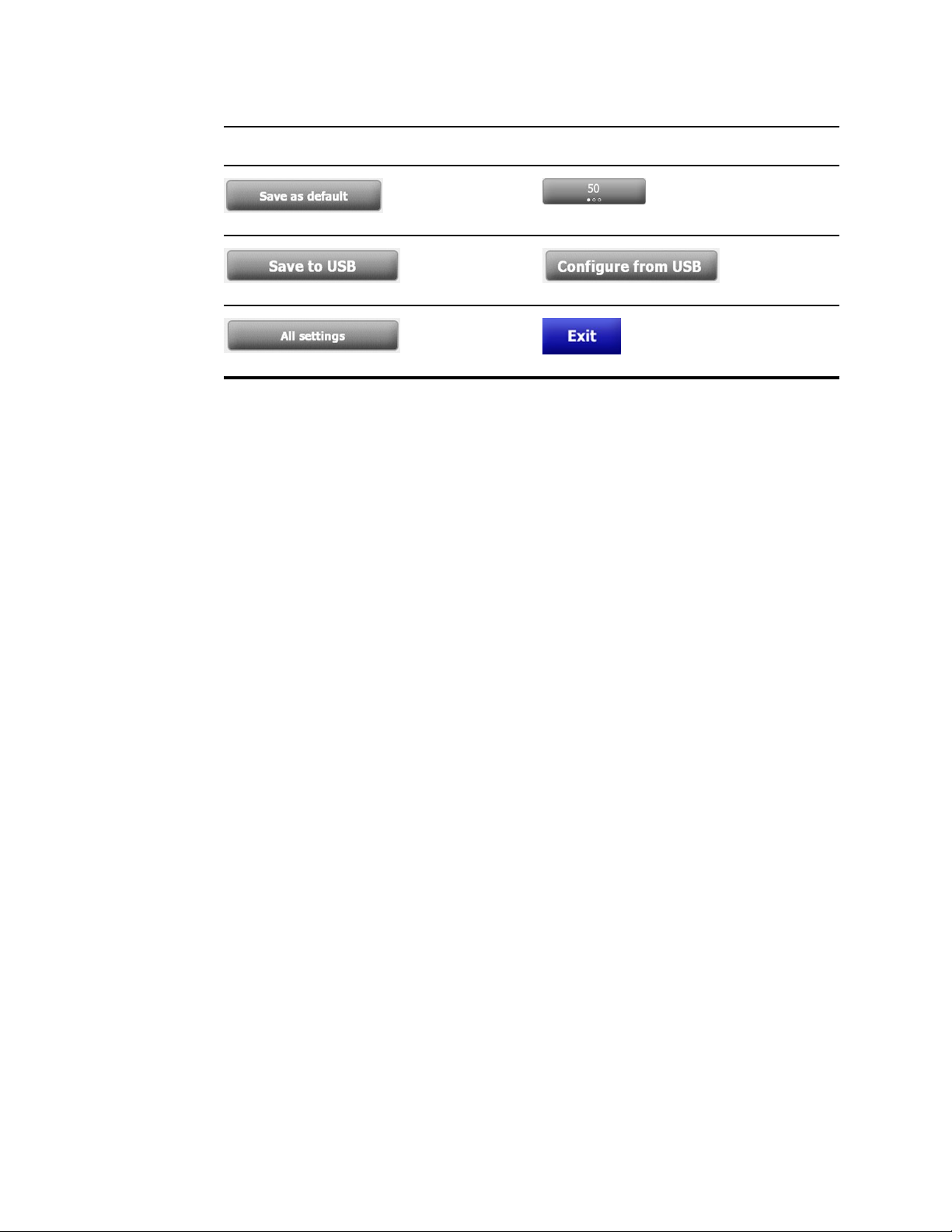
Settings
Save configuration
settings
Save to USB flash
drive
Restore factory
default settings
Select state or view
Configure from USB
flash drive
Close Advanced
settings

14 Screen elements Welch Allyn Connex® Devices
About warnings and cautions
Warning and caution statements can appear on the monitor, on the packaging, on the
shipping container, or in this document.
The monitor is safe for patients and clinicians when used in accordance with the
instructions and the warning and caution statements presented in this manual.
Before using the monitor, you must familiarize yourself with all warnings and cautions,
with the steps to power up the monitor, and with the sections of this directions for use
that pertain to your use of the monitor. In addition to reviewing the general warnings and
cautions presented in the next section, you must also review the more specific warnings
and cautions appear throughout the manual in conjunction with setup/startup, device
operation, patient monitoring, and maintenance tasks.
•
Failure to understand and observe any warning statement in this manual could lead
to patient injury, illness, or death.
• Failure to understand and observe any caution statement in this manual could lead to
damage to the equipment or other property, or loss of patient data.
15
General warnings and cautions
WARNING Many environmental variables, including patient physiology and
clinical application, can affect the accuracy and performance of the monitor.
Therefore, you must verify all vital signs information, especially NIBP and
SpO2, before treating the patient. If there is any question about the
accuracy of a measurement, verify the measurement using another
clinically accepted method.
WARNING Inaccurate measurement risk. Do not use the device or
accessories in environments affected by extremes of temperature,
humidity, or altitude. See "Environmental specifications" for acceptable
operating conditions.
WARNING Alarm limits are patient-specific. For alarms to function
properly, you must set or verify alarm limits appropriate for each patient.
Each time the monitor is powered on, you must check that the alarm
settings are appropriate for your patient before you start monitoring.
WARNING The monitor is not intended for use during patient transport
outside of the medical facility. Do not use the monitor to take
measurements on any patient in transit.

16 About warnings and cautions Welch Allyn Connex® Devices
WARNING Do not use the monitor as an apnea monitor. Neither the VSM
6000 series monitors, nor any of the integrated or accessory sensor
systems used in conjunction with the VSM 6000 series monitors, are
intended for use in apnea monitoring.
WARNING Use only Welch Allyn approved accessories, and use them
according to the manufacturer’s directions for use. Using unapproved
accessories with the monitor can affect patient and operator safety and can
compromise product performance and accuracy.
WARNING Inaccurate measurement risk. Do not connect more than one
patient to a monitor.
WARNING Inaccurate measurement risk. Dust and particle ingress can
affect the accuracy of blood pressure measurements. Use the monitor in
clean environments to ensure measurement accuracy. If you notice dust or
lint build-up on the monitor's vent openings, have the monitor inspected
and cleaned by a qualified service technician.
WARNING Inaccurate measurement risk. Liquids and excessive moisture
can damage patient sensors and cause them to perform inaccurately or fail.
WARNING Patient harm risk. Always remove sensors from patients and
disconnect them completely from monitors before bathing.
WARNING Liquids can damage electronics inside the monitor. Prevent
liquids from spilling on the monitor.
If liquids are spilled on the monitor:
1. Power down the monitor.
2. Disconnect the power plug.
3. Remove battery pack from the monitor.
4. Dry off excess liquid from the monitor.
Note If liquids possibly entered the monitor, remove the monitor
from use until it has been properly dried, inspected, and
tested by qualified service personnel.
5. Reinstall battery pack.
6. Power on the monitor and verify that the monitor functions normally before
using it.
If liquids enter the printer housing:
1. Power down the monitor.
2. Disconnect the power plug.
3. Remove battery pack from the monitor.
4. Remove and discard the paper roll.
5. Clean and dry the inside of the printer housing.
Note The printer housing has a drain tube that directs liquids down
and out the bottom of the monitor. If liquids possibly entered
other openings in the monitor, remove the monitor from use
until it has been properly dried, inspected, and tested by
qualified service personnel.
6. Install a new roll of paper.
7. Power on the monitor and verify that the monitor functions normally before
using it.

Directions for use About warnings and cautions 17
WARNING Safety risk and potential shock hazard. Cords, cables, and
accessories damaged from prior misuse can affect patient and operator
safety. Inspect all cords, cables, and accessories for strain relief wear,
fraying, or other damage according to the recommendations presented in
the Maintenance and service section of this manual. Replace as necessary.
Inspect the AC cord for exposed copper before touching the cord. Unplug
the AC cord only by pulling on the plug, never the cord. Never lift the
monitor by the power cord or patient connections.
WARNING Fire and explosion hazard. Do not operate the monitor in the
presence of a flammable anesthetic mixture with air, oxygen, or nitrous
oxide; in oxygen-enriched environments; or in any other potentially
explosive environment.
WARNING Fire and shock hazard. Only connect LAN cables contained
within the perimeter of a single building. Conductive LAN cables spanning
multiple buildings may introduce fire or shock hazards unless they are fitted
with fiber optic cables, lightning arrestors, or other applicable safety
features.
WARNING The monitor may not function properly if dropped or damaged.
Protect it from severe impact and shock. Do not use the monitor if you
notice any signs of damage. Qualified service personnel must check any
monitor that is dropped or damaged for proper operation before putting the
monitor back into use.
WARNING Defective batteries can damage the monitor. If the battery
shows any signs of damage or cracking, it must be replaced immediately
and only with a battery approved by Welch Allyn.
WARNING Improper disposal of batteries may create an explosion or
contamination hazard. Never dispose of batteries in refuse containers.
Always recycle batteries according to local regulations.
WARNING Electric shock hazard. Do not open the monitor or attempt
repairs. The monitor has no user-serviceable internal parts. Only perform
routine cleaning and maintenance procedures specifically described in this
manual. Never perform maintenance or service tasks while the device is
connected to a patient. Inspection and servicing of internal parts shall only
be performed by qualified service personnel.
WARNING Electric shock hazard. Never perform maintenance or service
tasks while the device is connected to a patient.
WARNING Inaccurate measurement risk. Do not expose to temperatures
higher than 122º F (50º C).
WARNING Inaccurate measurement risk. Do not use the monitor on
patients who are on heart-lung machines.
WARNING Inaccurate measurement risk. If using patient sensors during
full body irradiation, keep the sensor out of the irradiation field. If the
sensor is exposed to the irradiation, the reading might be inaccurate or the
monitor might read zero during the active irradiation period.
WARNING Inaccurate measurement risk. Do not use the monitor on
patients who are experiencing convulsions or tremors.

18 About warnings and cautions Welch Allyn Connex® Devices
WARNING Use the monitor only as described in this directions for use. Do
not use the monitor on patients as described in the Contraindications.
WARNING Personal/patient injury risk. Wall-mounted equipment and
accessories must be installed in accordance with accompanying
instructions. Improper installation can result in the monitor falling off the
wall and injuring someone. Welch Allyn is not responsible for the integrity
of any installation not performed by authorized Welch Allyn service
personnel. Contact an authorized Welch Allyn service representative or
other qualified service personnel to ensure professional installation for
safety and reliability of any mounting accessory.
WARNING Do not place the monitor in any position that might cause it to
fall on the patient.
WARNING Welch Allyn is not responsible for the integrity of a facility's
power. If the integrity of a facility's power or protective earth conductor is
in doubt, always operate the monitor on battery power alone when it is
attached to a patient.
WARNING Avoid continuously monitoring a patient when the device is
operating on battery power. If only battery power is available, you must
remain in the room with any patient whose vital signs are being monitored
continuously. Actively monitor both patient and battery status to ensure
patient safety.
WARNING Patient harm and equipment damage risk. Carefully route
patient cabling to reduce the possibility of patient entanglement or
strangulation. When transporting the monitor on a mobile stand, properly
secure all patient cables and cords to keep them clear of the wheels and to
minimize trip hazards.
WARNING For operator and patient safety, peripheral equipment and
accessories that can come in direct patient contact must comply with all
applicable safety, EMC, and regulatory requirements.
WARNING All signal input and output (I/O) connectors are intended for
connection of only devices complying with IEC 60601-1, or other IEC
standards (for example, IEC 60950), as applicable to the monitor.
Connecting additional devices to the monitor may increase chassis or
patient leakage currents. To maintain operator and patient safety, consider
the requirements of IEC 60601-1. Measure the leakage currents to confirm
that no electric shock hazard exists.
WARNING Equipment failure and patient harm risk. Do not cover the air
intake or exhaust vents on the rear and base of the monitor. Covering
these vents could cause overheating of the monitor or muffling of alarms.
WARNING Cross-contamination or nosocomial infection risk. Clean and
disinfect the monitor on a routine basis according to your facility's protocols
and standards or local regulations. Thorough hand-washing before and after
contact with patients greatly reduces the risk of cross-contamination and
nosocomial infection.
WARNING For patient safety, do not use the monitor or any accessory
during MRI scanning. Induced current could cause burns.
Directions for use About warnings and cautions 19
WARNING When the monitor is not conected to a secondary alarm
system during continuous monitoring, check the monitor regularly to
receive patient data, alarms, and alerts.
WARNING Patient safety risk. The EarlySense system is not intended for
high-risk patients in coronary or respiratory distress who require continuous
monitoring of heart function or CO2. For these patients, the most reliable
method of patient monitoring involves close personal surveillance and/or
equipment suited to that type of monitoring.
WARNING Patient injury risk. The decision to use the NIBP module on
pregnant or pre-eclamptic patients is at the discretion of the trained
clinician using the equipment.
WARNING Patient injury risk: The decision to use this device with
children, or pregnant or nursing women is at the discretion of the trained
clinician using the equipment.
WARNING Personal injury risk. The power cord is the disconnect device to
isolate this equipment from supply mains. Position the equipment so that it
is not difficult to reach to disconnect the cord.
WARNING Patient injury risk. This device is suitable for use with highfrequency surgical equipment but does not provide any additional means of
protection.
WARNING Safety risk and potential shock hazard. For accessories that
use a USB cable to connect to the monitor, disconnect the USB cable from
the monitor when you disconnect the accessory.
WARNING Personal/patient injury risk. Wall Mount must be mounted
using the appropriate hardware for the type of wall structure. Your facility
may need to provide the appropriate hardware needed to install for your
type of wall structure.
WARNING Personal/patient injury risk. Only authorized Welch Allyn
service personnel or a Biomedical engineer should attach or remove the
device from the wall mount.
WARNING Personal/patient injury risk. Any modification made to a Welch
Allyn mounting solution removes Welch Allyn from responsibility or liability
and voids the warranty.
WARNING Personal/patient injury risk. Welch Allyn is not responsible for
the integrity of any installation not performed by authorized Welch Allyn
service personnel.
WARNING Personal/patient injury risk. Welch Allyn is not responsible for
the integrity of any wall structure or wall mounting interface. Welch Allyn
recommends that you contact your Biomedical Engineering Department or
maintenance service to ensure professional installation, safety, and
reliability of any mounting accessory.
CAUTION Position the wall mount so that the screen, controls, and
connectors are accessible and support optimal and ergonomic use of the
device.

20 About warnings and cautions Welch Allyn Connex® Devices
CAUTION United States Federal law restricts this monitor to sale,
distribution, or use by or on the order of a physician or licensed healthcare
professional.
CAUTION Electromagnetic interference risk. The monitor complies with
applicable domestic and international standards for electromagnetic
interference. These standards are intended to minimize medical equipment
electromagnetic interference. Although this monitor is not expected to
present problems to other compliant equipment or be affected by other
compliant devices, interference issues still may occur. As a precaution,
avoid using the monitor in close proximity to other equipment. In the event
that equipment interference is observed, relocate the equipment as
necessary or consult manufacturer's directions for use.
CAUTION Use only a Class I (grounded) AC power supply cord for
powering this monitor.
CAUTION Do not use a long press of to power down the monitor when
it is functioning normally. You will lose patient data and configuration
settings.
CAUTION Never move the monitor or mobile stand by pulling on any of
the cords as this may cause the monitor to tip over or may damage the
cord. Never pull on the power cord when removing it from the power
outlet. When disconnecting the power cord, always grasp the attachment
plug and not the cord. Keep the cord away from liquids, heat, and sharp
edges. Replace the power cord if the strain relief or cord insulation is
damaged or begins to separate from the attachment plug.
CAUTION Use only the Welch Allyn USB client cable to connect a laptop
computer to the USB client port. Any laptop connected to the monitor must
be running on a battery, a 60601-1-compliant power supply, or a 60601-1compliant isolation transformer. While monitoring a patient, you can only
charge the laptop battery if it is connected to 60601-1-compliant, isolated
AC power.
CAUTION If the touchscreen is not responding properly, refer to the
troubleshooting section. If the problem cannot be resolved, discontinue use
of the monitor and contact an authorized Welch Allyn service center or
qualified service personnel.
CAUTION Verify patient identity on the monitor after manual or barcode
entry and before printing or transferring patient records.
CAUTION Keep the monitor outside of MRI suites and any areas marked
for high magnetic or electric field strength.
CAUTION Do not use the Suretemp to take or monitor the patient’s
temperature during defibrillation or electrosurgery. This may damage the
temperature probe.
CAUTION Before weighing a patient on any weight scale connected to the
monitor, disconnect all sensors from the patient. Doing so ensures an
accurate weight measurement and significantly reduces electrostatic
discharges that might disrupt the monitor.

Directions for use About warnings and cautions 21
Integrated Wall System warnings and cautions
In addition to the preceding warnings and cautions, consider the following when using
the Integrated Wall System.
WARNING Liquids can damage electronics inside the Connex IWS.
Prevent liquids from spilling on the wall system.
If liquids are spilled on the wall system:
1. Power down the wall system.
2. Disconnect the power plug.
3. Remove the wall system from the wall.
4. Remove battery pack from the wall system.
5. Dry off excess liquid from the wall system.
Note If liquids possibly entered the wall system, remove the wall
system from use until it has been properly dried, inspected,
and tested by qualified service personnel.
6. Reinstall battery pack.
7. Mount the wall system on the wall.
8. Power on the wall system and verify that it functions normally before using
it.
WARNING The physical assessment instruments (handles) are designed
for intermittent use. On-time should not exceed 2 minutes. Allow at least
10 minutes off-time between patients.
CAUTION Welch Allyn is not responsible for the integrity of any wall
mounting interface. Welch Allyn recommends that you contact your
Biomedical Engineering Department or maintenance service to ensure
professional installation, safety, and reliability of any mounting accessory.
CAUTION Do not use the Suretemp to take or monitor the patient’s
temperature during defibrillation or electrosurgery. This may damage the
temperature probe.

22 About warnings and cautions Welch Allyn Connex® Devices
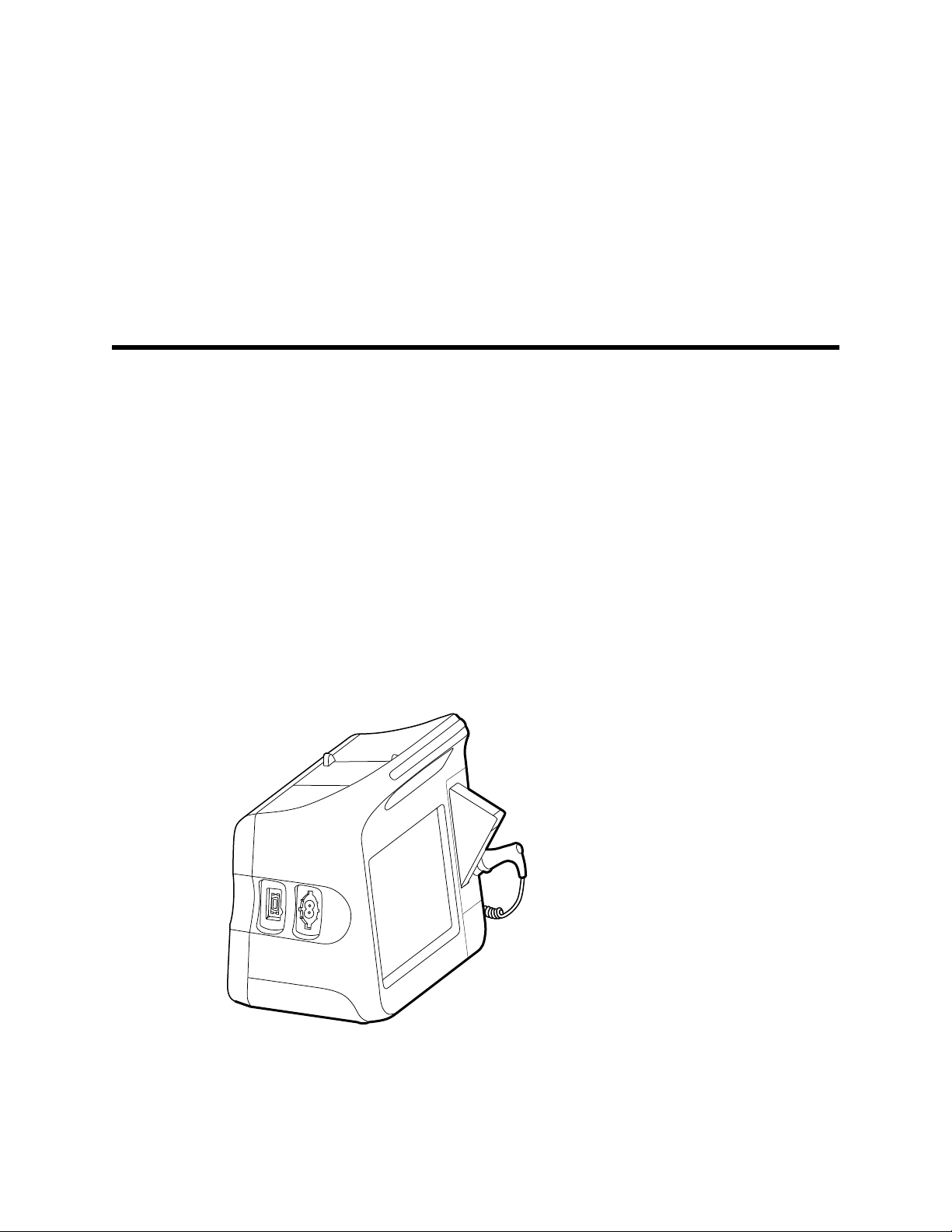
Physical design
Vital Signs Monitor 6000 Series
Standard and extended monitors
The monitor is available in two sizes: standard and extended. The primary difference
between these models is the number of parameters they support.
23
Note Your monitor, based on size or configuration, might not contain all
parameters or features illustrated in this section.
In a standard monitor, up to two parameter modules are installed on the left side. You
can tell which modules are installed based on the connectors visible on the outside of
the device. The following image shows a standard monitor with pulse-oximetry and
blood-pressure modules.
Standard monitor left side
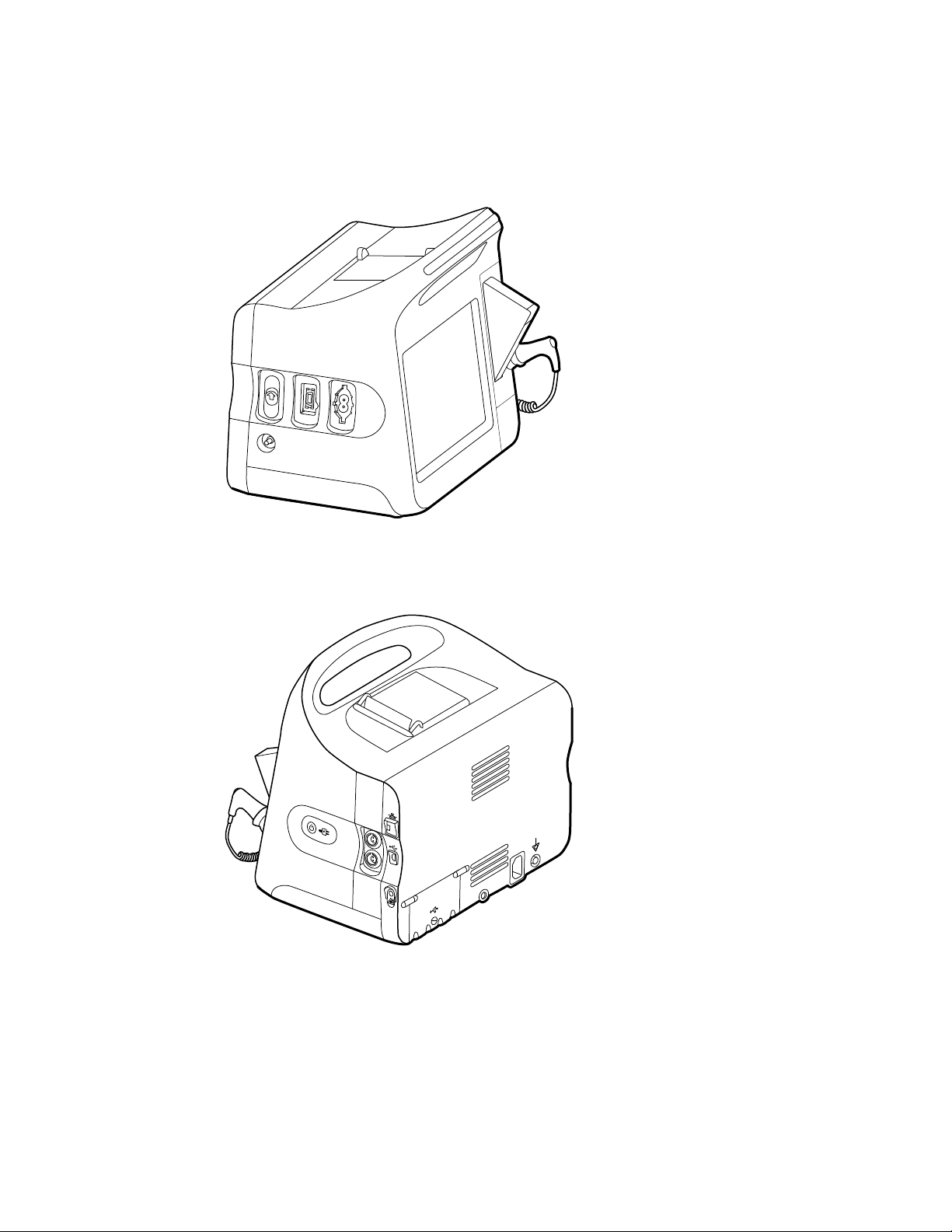
24
Physical design Welch Allyn Connex® Devices
The extended version can have a maximum of three modules (such as CO2, pulse
oximetry, and blood pressure) on the left side.
Extended monitor left side
If the extended monitor is configured with EarlySense, it has an additional module on the
right side.
Extended monitor right side
Equipment setup and basic monitor functions are the same for both models, unless
otherwise noted in the directions for use.
Controls, indicators, and connectors
The following diagrams show a full-featured monitor. Your monitor, based on size or
configuration, might not contain all of these features.
 Loading...
Loading...