GE Dash 3000, Dash 4000, Dash 5000 User manual

Dash® 3000/4000/5000
Patient Monitor
Operator's Manual
2023896-026 |
Revision A |

NOTE
Due to continuing product innovation, specifications in this manual are subject to change without notice. The information in this manual supports software versions 5.4 or later.
Listed below are GE Medical Systems Information Technologies trademarks used in this document. All other trademarks contained herein are the property of their respective owners.
CD TELEMETRY, CRG PLUS, DASH, DINAMAP, EAGLE, MARS, MUSE, RESPONDER, SAM, SOLAR, TRAMNET, TRAMSCOPE, TRAM-RAC, TRIM KNOB, UNITY and UNITY NETWORK are trademarks of GE Medical Systems Information Technologies registered in the United States Patent and Trademark Office.
12SL, CENTRALSCOPE, CIC PRO, DASH PORT, EK-PRO, IMPACT, INTELLIRATE, MENTOR, PRISM, PRN 50-M and SUPERSTAT are trademarks of GE Medical Systems Information Technologies.
© 2005 General Electric Company. All rights reserved.
T-2 |
Dash® 3000/4000/5000 |
2000966-338A |
|
|
10 May 2005 |

Contents
1 |
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-1 |
|
Equipment Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-2 |
|
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.1-2 |
|
Safety Statements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-2 |
|
Dangers. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.1-2 |
|
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.1-2 |
|
Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.1-8 |
|
Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-13 |
|
Equipment Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-14 |
|
Equipment Compliance Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-15 |
|
EN 60601-1 Component Classification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-15 |
|
Underwriters Laboratories, Inc. Classification . . . . . . . . . . . . . . . . . . . . . . . . |
1-16 |
|
AAMI EMI Environment Recommendations . . . . . . . . . . . . . . . . . . . . . . . . . |
1-16 |
|
FCC Compliance Information Statement. . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-17 |
|
Manual Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-17 |
|
Purpose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-17 |
|
Intended Audience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-17 |
|
Conventions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-18 |
|
Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-18 |
|
User Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-18 |
|
Text . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-19 |
|
Illustrations and Names. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-19 |
|
Ordering Manuals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-20 |
|
Revision History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-20 |
2 |
Equipment Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-1 |
|
Hardware Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-2 |
|
Front . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-2 |
|
Right . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-3 |
|
Left . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-4 |
|
Back . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-5 |
|
Control Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-6 |
|
Trim Knob Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-8 |
|
Indicators. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-9 |
|
Alarm Light . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-9 |
|
Power . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-9 |
|
Charging Status. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-9 |
|
Battery Status . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.2-9 |
|
Optional Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-10 |
|
TRAM-RAC 2A Module Housing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-10 |
|
Nellcor® 395 Pulse Oximeter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-10 |
|
Unity Network . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-10 |
|
Centralscope Central Station . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-11 |
2000966-338A |
Dash® 3000/4000/5000 |
i |

CIC Pro Clinical Information Center . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-11
Wireless LAN System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-11
Laser Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12
PRN 50-M Digital Writer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12
Remote Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12
Dash Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Remote Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Cadex® SMart Two+ Charger . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Operating Modes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-14
Normal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Off/Charging . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
User Interface Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-16
Main Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-16
Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-17
Windows . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-17
3 |
Monitor Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-1 |
|
Getting Started . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-2 |
|
Identifying Your Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-2 |
|
Software Packages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-2 |
|
Software Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-3 |
|
Monitoring/Admit Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-3 |
|
Patient-Monitor Type . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-4 |
|
Language . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-4 |
|
Locale . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-5 |
|
France . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-5 |
|
Customizing Monitor Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-6 |
|
Monitor Defaults Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-6 |
|
Factory Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-6 |
|
Custom Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-6 |
|
Control Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-7 |
|
Defining Custom Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-7 |
|
Defining Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.3-9 |
|
Restoring Factory Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-12 |
|
Defining Parameter Window Priority . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-12 |
4 |
Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.4-2 |
|
Alarm Types . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-3 |
|
Patient Status Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.4-3 |
|
System Status Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.4-4 |
|
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-4 |
ii |
Dash® 3000/4000/5000 |
2000966-338A |

|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-5 |
|
Silencing Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-6 |
|
Temporarily . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.4-6 |
|
Permanently . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.4-8 |
5 |
Managing Patients . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-2 |
|
Admit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.5-2 |
|
Standard Admit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.5-2 |
|
Quick Admit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.5-3 |
|
Automatic Admit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.5-3 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.5-3 |
|
Discharge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-5 |
|
View Other Patients . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-6 |
|
Software Option Comparison . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.5-6 |
|
Limitations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.5-7 |
|
Custom Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.5-8 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-10 |
|
Viewing Other Monitors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-11 |
|
Selecting a Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-11 |
|
Understanding the Split-View Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-12 |
|
Removing a Viewed Monitor Display. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-13 |
6 |
Printing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.6-2 |
|
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.6-2 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-2 |
|
Printed Output . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-3 |
|
Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-4 |
7 |
Patient Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-2 |
|
Reviewing Patient Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-2 |
|
Alarm History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.7-2 |
|
Vital Signs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.7-4 |
|
Graphic Trends . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.7-5 |
2000966-338A |
Dash® 3000/4000/5000 |
iii |

CRG Trends . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-7
Lab Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-9
Customizing the Trends Key . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-10 |
Calculations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-10 |
Cardiac Calculations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-10 |
Dosage Calculations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-11 |
Pulmonary Calculations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-13 |
8 |
Monitoring ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.8-2 |
|
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-3 |
|
AFIB Arrhythmia Detection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.8-3 |
|
IntelliRate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.8-5 |
|
Combo and Rover Combo Monitoring Guidelines . . . . . . . . . . . . . . . . . . . . . . . . |
.8-6 |
|
Analog Output . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.8-7 |
|
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.8-7 |
|
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-8 |
|
Pacemaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.8-8 |
|
Arrhythmia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.8-9 |
|
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-10 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-11 |
|
Custom Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-14 |
|
Turning Off ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-14 |
|
Permanently . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-14 |
|
Constraints . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-15 |
|
ECG Setting Source . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-15 |
|
Selecting ECG Setting Source . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-15 |
|
ECG Setting Source When Entering COMBO Mode. . . . . . . . . . . . . . . . . . . |
8-16 |
|
ECG Setting Source When Exiting COMBO Mode . . . . . . . . . . . . . . . . . . . . |
8-17 |
|
ECG Rate Averaging . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-17 |
|
IntelliRate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-17 |
|
AFIB Alarm Level . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-18 |
|
Performing ST Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-18 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-18 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-19 |
|
Performing 12 Lead Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-21 |
|
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-23 |
iv |
Dash® 3000/4000/5000 |
2000966-338A |

9 |
Monitoring Invasive Pressures . . . . . . . . . . . . . . . . . . . . . |
9-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.9-2 |
|
Pressure Site Names and Values . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.9-3 |
|
Default Site Labels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.9-3 |
|
Analog Output . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.9-3 |
|
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.9-4 |
|
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-4 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-5 |
|
Performing PA Wedge Measurements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-7 |
|
Automatic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.9-7 |
|
Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.9-9 |
|
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-9 |
10 |
Monitoring NBP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 10-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. .10-2 |
|
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.10-3 |
|
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.10-4 |
|
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-4 |
|
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-7 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-7 |
|
Custom Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-10 |
|
Setup Custom Automatic NBP Measurements . . . . . . . . . . . . . . . . . . . . . . . . . |
10-10 |
|
Auto NBP Cancellation Notification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-11 |
|
Extended NBP Alarm Silence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-11 |
|
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
10-12 |
11 |
Monitoring SpO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
11-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 11-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. .11-2 |
|
Primary Parameter Monitoring Considerations . . . . . . . . . . . . . . . . . . . . . . . . . |
.11-3 |
|
Configuration Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
11-3 |
|
Masimo . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.11-3 |
|
Nellcor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.11-4 |
|
Ohmeda. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.11-5 |
|
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.11-6 |
2000966-338A |
Dash® 3000/4000/5000 |
v |

Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-7
Neonates and Infants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-9
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-9
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-10
Nellcor 395 Pulse Oximeter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-13
Connecting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-13
Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-15
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-16
12 Measuring Cardiac Output . . . . . . . . . . . . . . . . . . . . . . . .12-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12-2
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-2
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-2
Measuring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12-3
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12-3
Performing the CO Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12-5
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12-7
13 |
Monitoring Respiration . . . . . . . . . . . . . . . . . . . . . . . . . . . |
13-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 13-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. .13-2 |
|
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.13-3 |
|
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
13-3 |
|
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
13-3 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
13-4 |
|
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
13-5 |
14 |
Monitoring Temperature . . . . . . . . . . . . . . . . . . . . . . . . . . |
14-1 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 14-2 |
|
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 14-2 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
14-3 |
vi |
Dash® 3000/4000/5000 |
2000966-338A |

Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
15 Monitoring End-Tidal CO2 . . . . . . . . . . . . . . . . . . . . . . . .15-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-2
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15-2
Compatible Devices/Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15-2
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15-3
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-3
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-4
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-5
Calibrating . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-6
Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15-6
Adapters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15-7
Sample Lines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15-7
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-8
16 |
Monitoring Anesthesia Gases . . . . . . . . . . . . . . . . . . . . . |
16-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 16-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. .16-2 |
|
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.16-3 |
|
Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.16-3 |
|
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.16-3 |
|
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
16-3 |
|
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
16-6 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
16-6 |
|
CO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.16-6 |
|
Gas . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.16-8 |
|
Enabling HAL and ENF . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.16-9 |
|
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
16-9 |
17 |
Monitoring Impedance Cardiography (ICG) . . . . . . . . . . |
17-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 17-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. .17-2 |
|
Monitored Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.17-2 |
|
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.17-2 |
|
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
17-3 |
2000966-338A |
Dash® 3000/4000/5000 |
vii |

|
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 17-4 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
17-5 |
|
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
17-7 |
18 |
Monitoring Bispectral Index (BIS) . . . . . . . . . . . . . . . . . . |
18-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-2 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-2 |
|
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-3 |
|
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-3 |
|
Considerations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-3 |
|
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-3 |
|
Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-4 |
|
Control Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-5 |
|
Disabling Continuous Lead Detection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-6 |
|
Disabling the Waveform Filter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-7 |
|
Testing the BIS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-7 |
|
Understanding Displayed Values . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-7 |
|
BIS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-7 |
|
SR . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-8 |
|
SQI . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-8 |
|
SEF . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-8 |
|
EMG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
.18-8 |
|
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
18-8 |
A |
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
A-1 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-2 |
|
Biocompatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-2 |
|
Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-2 |
|
Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-2 |
|
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-3 |
|
Exterior Surface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-3 |
|
Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-4 |
|
Applied Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-4 |
|
Cables and Leadwires. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-4 |
|
Capnostat Sensor and Adapter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-4 |
|
NBP Cuff and Hose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-5 |
|
Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. A-5 |
viii |
Dash® 3000/4000/5000 |
2000966-338A |

Printer/Writer(s). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-5
Changing Writer Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-5
Batteries. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-6
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-6
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-6
Lithium-Ion Technology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-7
Cadex SMart Two+ Battery Charger . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-7
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-8
Identifying Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-9
Installation Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-10
Battery Care . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-11
Charging . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-11
Conditioning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-11
Storing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-12
Waking Up. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-13
Recycling. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-13
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-14
Technical Maintenance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-15
Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-15
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-15
Temperature Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-15
NBP Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-16
Alarm Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-18
B |
Software Packages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
B-1 |
|
Feature Comparison by Software Package . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. B-2 |
C |
Factory Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-1 |
|
Arrhythmia Alarm Levels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-2 |
|
Parameter Alarm Levels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-2 |
|
Parameter Limits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-4 |
|
Display Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-8 |
|
Default Parameter Priority . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
C-13 |
D |
Custom Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
D-1 |
|
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
D-2 |
E |
Interfacing with Non-GE Devices . . . . . . . . . . . . . . . . . . . |
E-1 |
|
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
E-2 |
2000966-338A |
Dash® 3000/4000/5000 |
ix |

General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-2
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-2
Non-GE Device Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-3
Displayed Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-3
Compatible Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-4
Limitations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-8
Duplicate Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-8
Duplicate Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-8
Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-9
Connecting the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-9
Connecting Peripheral Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-10
Establishing Communication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-11
Customizing Data Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-12
Default Parameter Alarm Levels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-12
Alarm Limits. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-13
Parameter Windows and Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-13
Peripheral Device Monitoring Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-13
Anesthesia Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-13
Gas Interface. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-13
Pulse Oximeter Interface. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-14
Transcutaneous pO2/pCO2 Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-14
Ventilator Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-14
Continuous Cardiac Output . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-14
IV Pump. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-14
Urometers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-15
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . E-15
F |
Abbreviations and Symbols . . . . . . . . . . . . . . . . . . . . . . . . |
F-1 |
|
Abbreviations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
F-2 |
|
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
F-9 |
x |
Dash® 3000/4000/5000 |
2000966-338A |

CE Marking Information
CE Marking Information
Compliance
The Dash® 3000/4000/5000 patient monitor bears CE mark CE-0459 indicating its conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive. The product is in radio-interference protection class A in accordance with
EN 55011.
The country of manufacture can be found on the equipment labeling.
The product complies with the requirements of standard EN 60601-1-2 “Electromagnetic Compatibility - Medical Electrical Equipment”.
The system meets the requirements of EN 60601-1-2 (2001) Medical Electrical
Equipment, Part 1: General Requirements for Safety, 2. Collateral Standard:
Electromagnetic compatibility — Requirements and tests.
Exceptions
Users should be aware of known RF sources, such as radio or TV stations and handheld or mobile two-way radios, and consider them when installing a medical device or system.
Be aware that adding accessories or components, or modifying the medical device or system may degrade the EMI performance. Consult with qualified personnel regarding changes to the system configuration.
Radio and Telecommunication Terminal Equipment Directive
The monitor contains a transmitter. The transmitter bears a CE mark indicating conformity with the essential requirements specified in Article 3 of the Council Directive 1999/5/EC of 9 March 1999 concerning Radio Equipment and Telecommunications Terminal Equipment (R&TTE).
The essential requirements are as follows:
Article 3.1 (a) Health Safety; the product complies with the particular medical device safety standards specified in the Medical Device Directive 93/42/EEC: EN 60601-1/1990 + A1: 1993 + A2: 1995: Medical electrical equipment. General requirements for safety.
Article 3.1 (b) EMC; the product complies with:
2000966-338A |
Dash® 3000/4000/5000 |
CE-1 |

CE Marking Information
EN 60601-1-2 (2001) Medical electrical equipment: Part 1: General requirements for safety - 2. Collateral standard: Electromagnetic compatibility - requirements and test.
ETS 300 826 (1997): “Electromagnetic compatibility and Radio spectrum Matters (ERM); ElectroMagnetic Compatibility (EMC) standard for 2.4 GHz wideband transmission systems and HIgh PErformance Radio Local Area Network (HIPERLAN) equipment”.
Article 3.2 Protection of the Radio Spectrum: the product complies with: ETSI EN 300 328 (2003): “Radio Equipment and Systems (RES); Wideband transmission systems; Technical characteristics and test conditions for data transmission equipment operating in the 2.4 GHz ISM band and using spread spectrum modulation techniques.
General Information
This manual is an integral part of the product and describes its intended use. It should always be kept close to the equipment. Observance of the manual is a prerequisite for proper product performance and correct operation and ensures patient and operator safety.
The symbol |
means ATTENTION: Consult accompanying documents. |
Information which refers only to certain versions of the product is accompanied by the model number(s) of the product(s) concerned. The model number is given on the nameplate of the product.
The warranty does not cover damages resulting from the use of accessories and consumables from other manufacturers.
GE is responsible for the effects on safety, reliability, and performance of the product, only if:
assembly operations, extensions, readjustments, modifications, or repairs are carried out by persons authorized by GE;
the electrical installation of the relevant room complies with the requirements of the appropriate regulations; and,
the device is used in accordance with the instructions for use.
All publications conform with the product specifications and applicable EN publications on safety and essential performance of electromedical equipment as well as with applicable UL and CSA requirements and AHA recommendations valid at the time of printing.
The quality management system complies with the international standards ISO 9001 and ISO 13485, and the Council Directive on Medical Devices 93/42/ EEC.
CE-2 |
Dash® 3000/4000/5000 |
2000966-338A |

1 Introduction
2000966-338A |
Dash® 3000/4000/5000 |
1-1 |

Introduction
Equipment Information
Intended Use
The intended use of this device is to monitor physiologic parameter data on adult, pediatric and neonatal patients within a hospital or facility providing patient care.
Physiologic data includes the parameters covered in this manual. The monitoring system is also intended to provide physiologic data over the optional network to clinical information systems. This information can be viewed, trended, stored and printed.
Safety Statements
The safety statements presented in this chapter refer to the equipment in general and, in most cases, apply to all aspects of the monitor. There are additional safety statements in the parameter chapters which are specific to that monitored parameter.
The order in which safety statements are presented in no way implies order of importance.
Dangers
Danger statements identify an imminent hazard which, if not avoided, will result in death or serious injury. No danger statements apply to this monitoring system.
Warnings
Warning statements identify a potential hazard or unsafe practice which, if not avoided, could result in death or serious injury.
The following warning statements apply to this monitoring system:
WARNING
ACCIDENTAL SPILLS — To avoid electric shock or device malfunction, liquids must not be allowed to enter the device. If liquids have entered a device, take it out of service and have it checked by a service technician before it is used again.
1-2 |
Dash® 3000/4000/5000 |
2000966-338A |

Introduction
WARNING
ACCURACY — If the accuracy of any value viewed on the monitor, central station, or printed on a graph strip is questionable, determine the patient's vital signs by alternative means. Verify that all equipment is working correctly.
WARNING
ALARMS — Do not rely exclusively on the audible alarm system for patient monitoring. Adjustment of alarm volume to a low level or off during patient monitoring may result in a hazard to the patient. Remember that the most reliable method of patient monitoring combines close personal surveillance with correct operation of monitoring equipment.
After connecting the monitor to the central station and/or nurse-call system, verify the function of the alarm system.
The functions of the alarm system for monitoring of the patient must be verified at regular intervals.
WARNING
BEFORE USE — Before putting the system into operation visually inspect all connecting cables for signs of damage. Damaged cables and connectors must be replaced immediately.
Before using the system, the operator must verify that it is in correct working order and operating condition.
Periodically, and whenever the integrity of the product is in doubt, test all functions.
WARNING
CABLES — Route all cables away from patient's throat to avoid possible strangulation.
2000966-338A |
Dash® 3000/4000/5000 |
1-3 |

Introduction
WARNING
CONDUCTIVE CONNECTIONS — Extreme care must be exercised when applying medical electrical equipment. Many parts of the human/machine circuit are conductive, such as the patient, connectors, electrodes, transducers. It is very important that these conductive parts do not come into contact with other grounded, conductive parts when connected to the isolated patient input of the device. Such contact would bridge the patient's isolation and cancel the protection provided by the isolated input. In particular, there must be no contact of the neutral electrode and ground.
WARNING
DEFIBRILLATION — Do not come into contact with patients during defibrillation. Otherwise serious injury or death could result.
WARNING
DISCHARGE TO CLEAR PATIENT DATA — When admitting a new patient, you must clear all previous patient data from the system. To accomplish this, disconnect patient cables then do a discharge.
WARNING
DISCONNECTION FROM MAINS — When disconnecting the system from the power line, remove the plug from the wall outlet first. Then you may disconnect the power cord from the device. If you do not observe this sequence, there is a risk of coming into contact with line voltage by inserting metal objects, such as the pins of leadwires, into the sockets of the power cord by mistake.
WARNING
DISPOSAL — Dispose of packaging material, observing the applicable waste control regulations and keeping it out of children’s reach.
1-4 |
Dash® 3000/4000/5000 |
2000966-338A |

Introduction
WARNING
EXPLOSION HAZARD — Do not use this equipment in the presence of flammable anesthetics, vapors or liquids.
WARNING
WIRELESS COMMUNICATION INTERFERENCE — Wireless LAN equipment contains an intentional RF radiator that has the potential of interfering with other medical equipment, including patient implanted devices.
Before installation and any time new medical equipment is added to the Wireless LAN coverage area, complete the following tests:
Software version 5.3 or earlier — Perform the electromagnetic compatibility test as described in the Wireless LAN Configuration Guide.
Software version 5.4 or later — Perform the ad-hoc tests AND the electromagnetic compatibility test as described in the Wireless LAN Configuration Guide.
WARNING
INTERFACING OTHER EQUIPMENT — Devices may only be interconnected with each other or to parts of the system when it has been determined by qualified biomedical engineering personnel that there is no danger to the patient, the operator, or the environment as a result. In those instances where there is any element of doubt concerning the safety of connected devices, the user must contact the manufacturers concerned (or other informed experts) for proper use. In all cases, safe and proper operation should be verified with the applicable manufacturer's instructions for use, and system standard EN 60601-1-1 must be complied with.
WARNING
— Before using the monitor for the first time, please read the “Safety Statements” on page 1-2.
2000966-338A |
Dash® 3000/4000/5000 |
1-5 |

Introduction
WARNING
INTRACARDIAC APPLICATION — When applying devices intracardially, electrically conductive contact with parts connected to the heart (pressure transducers, metal tube connections and stopcocks, guide wires, etc.) must be avoided in all cases.
To prevent electrical contact, we recommend the following:
Always wear isolating rubber gloves.
Keep parts that are conductively connected to the heart isolated from the ground.
Do not use tube fittings or stopcocks made of metal.
During intracardiac application of a device, a defibrillator and pacemaker whose proper functioning has been verified must be kept at hand.
WARNING
LEAKAGE CURRENT TEST — When interfacing with other equipment, a test for leakage current must be performed by qualified biomedical engineering personnel before using with patients.
WARNING
PATIENT AMBULATION — A patient must be assisted if ambulating with a roll-stand mounted monitor.
WARNING
POWER SUPPLY — The device must be connected to a properly installed power outlet with protective earth contacts only. If the installation does not provide for a protective earth conductor, disconnect the monitor from the power line and operate it on battery power, if possible.
All devices of a system must be connected to the same power supply circuit. Devices which are not connected to the same circuit must be electrically isolated when operated.
1-6 |
Dash® 3000/4000/5000 |
2000966-338A |
Introduction
WARNING
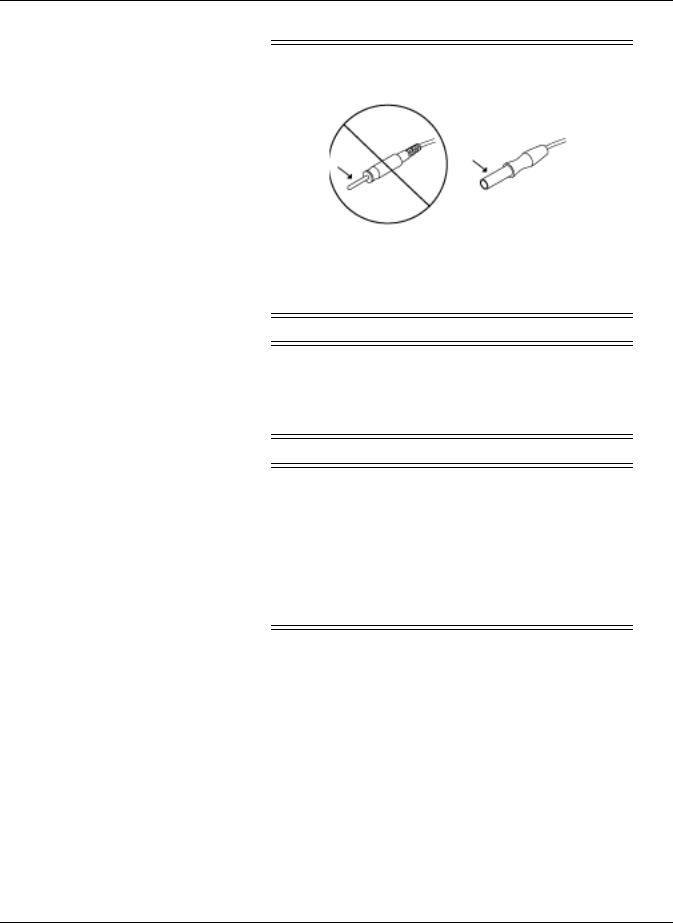
PROTECTED LEADWIRES — Only use protected leadwires and patient cables with this monitor.
The use of unprotected leadwires and patient cables creates the potential for making an electrical connection to ground or to a high voltage power source which can cause serious injury or death to the patient.
WARNING
RATE METERS — Keep pacemaker patients under close observation. Rate meters may continue to count the pacemaker rate during cardiac arrest and some arrhythmias. Therefore, do not rely entirely on rate meter alarms.
WARNING
SITE REQUIREMENTS — For safety reasons, all connectors for patient cables and sensor leads are designed to prevent inadvertent disconnection, should someone pull on them.
Do not route cables in a way that they may present a stumbling hazard.
For devices installed above the patient, adequate precautions must be taken to prevent them from dropping on the patient.
2000966-338A |
Dash® 3000/4000/5000 |
1-7 |

Introduction
WARNING
— If an error message appears DURING operation, it is the physician’s responsibility to decide whether the unit is still suitable for patient monitoring. As a general rule, monitoring should only continue in extremely urgent cases and under the direct supervision of a physician. The unit must be repaired before being used again on a patient.
If an error message appears AFTER power-up, the unit must be repaired before being used on a patient.
WARNING
— If connecting a monitor to a central station, verify the alarm system is functional by temporarily changing one of the alarm limits to initiate an alarm.
Cautions
Caution statements identify a potential hazard or unsafe practice which, if not avoided, could result in minor personal injury or product/property damage.
The following caution statements apply to this monitoring system:
CAUTION
ACCESSORIES (SUPPLIES) — To ensure patient safety, use only parts and accessories manufactured or recommended by GE.
Parts and accessories used must meet the requirements of the applicable EN 60601 series safety standards and essential performance standards, and/or the system configuration must meet the requirements of the EN 60601-1-1 medical electrical systems standard.
CAUTION
ACCESSORIES (EQUIPMENT) — The use of ACCESSORY equipment not complying with the equivalent safety requirements of this equipment may lead to a reduced level of safety of the resulting system. Consideration relating to the choice shall
1-8 |
Dash® 3000/4000/5000 |
2000966-338A |

Introduction
include:
use of the accessory in the PATIENT VICINITY; and
evidence that the safety certification of the ACCESSORY has been performed in accordance to the appropriate EN 60601-1 and/or EN 60601-1-1 harmonized national standard.
CAUTION
BATTERY POWER — If a device equipped with an optional battery pack will not be used or not be connected to the power line for a period of over six months, remove the battery.
CAUTION
BEFORE INSTALLATION — Compatibility is critical to safe and effective use of this device. Please contact your local sales or service representative prior to installation to verify equipment compatibility.
CAUTION
CO2 PARAMETER INTERFERENCE — Capnostat CO2
sensors with a serial number 26104 or greater require a separation distance of 0.25 meters (10 inches) from the Wireless LAN device to minimize potential interference.
CO2 sensors with a serial number less than 26104 require a
separation distance of 2.5 meters (8.2 feet) and are not recommended for use on monitoring equipment equipped with the Wireless LAN option.
CAUTION
DEFIBRILLATOR PRECAUTIONS — Patient signal inputs labeled with the CF and BF symbols with paddles are protected against damage resulting from defibrillation voltages. To ensure proper defibrillator protection, use only the recommended cables and leadwires.
Proper placement of defibrillator paddles in relation to the electrodes is required to ensure successful defibrillation.
2000966-338A |
Dash® 3000/4000/5000 |
1-9 |

Introduction
CAUTION
DISPOSABLES — Disposable devices are intended for single use only. They should not be reused as performance could degrade or contamination could occur.
CAUTION
DISPOSAL — At the end of its service life, the product described in this manual, as well as its accessories, must be disposed of in compliance with the guidelines regulating the disposal of such products. If you have questions concerning disposal of the product, please contact GE or its representatives.
CAUTION
ELECTROCAUTERY PRECAUTIONS — To prevent unwanted skin burns, apply electrocautery electrodes as far as possible from all other electrodes, a distance of at least 15 cm/6 in. is recommended.
CAUTION
ELECTRODES — Whenever patient defibrillation is a possibility, use non-polarizing (silver/silver chloride construction) electrodes for ECG monitoring. Polarizing electrodes (stainless steel or silver constructed) may cause the electrodes to retain a residual charge after defibrillation. A residual charge will block acquisition of the ECG signal.
CAUTION
EMC — Magnetic and electrical fields are capable of interfering with the proper performance of the device. For this reason make sure that all external devices operated in the vicinity of the monitor comply with the relevant EMC requirements. X-ray equipment or MRI devices are a possible source of interference as they may emit higher levels of electromagnetic radiation.
1-10 |
Dash® 3000/4000/5000 |
2000966-338A |

Introduction
CAUTION
INSTRUCTIONS FOR USE — For continued safe use of this equipment, it is necessary that the listed instructions are followed. However, instructions listed in this manual in no way supersede established medical practices concerning patient care.
CAUTION
LOSS OF DATA — Should the monitor at any time temporarily lose patient data, the potential exists that active monitoring is not being done. Close patient observation or alternate monitoring devices should be used until monitor function is restored.
If the monitor does not automatically resume operation within 60 seconds, power cycle the monitor using the power on/off switch. Once monitoring is restored, you should verify correct monitoring state and alarm function.
CAUTION
MAINTENANCE — Regular preventative maintenance should be carried out annually. You are responsible for any requirements specific to your country.
CAUTION
MPSO — The use of a multiple portable socket outlet (MPSO) for a system will result in an enclosure leakage current equal to the sum of all individual earth leakage currents of the system if there is an interruption of the MPSO protective earth conductor. Do not use an additional extension cable with the MPSO as it will increase the chance of the single protective earth conductor interruption.
CAUTION
NEGLIGENCE — GE does not assume responsibility for damage to the equipment caused by improperly vented cabinets, improper or faulty power, or insufficient wall strength to support equipment mounted on such walls.
2000966-338A |
Dash® 3000/4000/5000 |
1-11 |

Introduction
CAUTION
OPERATOR — Medical technical equipment such as this monitor/monitoring system must only be used by persons who have received adequate training in the use of such equipment and who are capable of applying it properly.
CAUTION
POWER REQUIREMENTS — Before connecting the device to the power line, check that the voltage and frequency ratings of the power line are the same as those indicated on the unit's label. If this is not the case, do not connect the system to the power line until you adjust the unit to match the power source.
In U.S.A., if the installation of this equipment will use 240V rather than 120V, the source must be a center-tapped, 240V, single-phase circuit.
This equipment is suitable for connection to public mains as defined in CISPR 11.
CAUTION
RESTRICTED SALE — U.S. federal law restricts this device to sale by or on order of a physician.
CAUTION
SINGLE PATIENT USE — This equipment is designed for use on one patient at a time. Using this equipment to monitor different parameters on different patients at the same time compromises the accuracy of data acquired.
CAUTION
SUPERVISED USE — This equipment is intended for use under the direct supervision of a licensed health care practitioner.
1-12 |
Dash® 3000/4000/5000 |
2000966-338A |

Introduction
CAUTION
VENTILATION REQUIREMENTS — Set up the device in a location which affords sufficient ventilation. The ventilation openings of the device must not be obstructed. The ambient conditions specified in the technical specifications must be ensured at all times.
Notes
Note statements provide application tips or other useful information.
The following note statements apply to this monitoring system:
Put the monitor in a location where you can easily see the screen and access the operating controls.
This product is not likely to cause abnormal operation of other patientconnected equipment such as cardiac pacemaker or other electrical stimulators. Exceptions are noted in the pacemaker monitoring section, if applicable.
This product is protected against the effects of cardiac defibrillator discharges to ensure proper recovery, as required by test standards. (The screen may blank during a defibrillator discharge but recovers within seconds as required by test standards.)
This equipment is suitable for use in the presence of electrosurgery.
Users should be aware of a possible time discrepancy between the waveforms from the telemetry device and the waveforms hardwired to the monitor. Users should not consider these waveforms to be synchronous. If absolute synchronicity is desired, COMBO mode should be discontinued and the ECG waveforms should be acquired via the hardwired bedside monitor.
The ambient conditions specified in the technical specifications of the service manual must be ensured at all times.
Connect the power cord supplied with the monitor. Use only the original cord or an equivalent one.
For measurements in or near the heart, GE recommends connecting the monitor to the equipotential stud. Use the green and yellow potential equalization cable and connect it to the equipotential stud on the back of the monitor.
Contact customer service engineers to connect monitors to a central station.
Monitors may be shipped with protective covers to protect unused parameter connector inputs from dust and liquids. Do not remove the protective covers; the parameter connectors will not function without them.
2000966-338A |
Dash® 3000/4000/5000 |
1-13 |
Introduction
Equipment Symbols
NOTE
Some symbols may not appear on all equipment.
ATTENTION: Consult accompanying documents.
CAUTION: To reduce the risk of electric shock, do NOT remove cover. Refer servicing to qualified service personnel.
TYPE CF APPLIED PART: Isolated (floating) applied part suitable for intentional external and internal application to the patient including direct cardiac application. “Paddles” outside the box indicate the applied part is defibrillator proof.
[Medical Standard Definition:] F-type applied part (floating/isolated) complying with the specified requirements of EN 60601-1/UL 60601-1/CSA 601.1 Medical Standards to provide a higher degree of protection against electric shock than that provided by type BF applied parts.
TYPE BF APPLIED PART: Isolated (floating) applied part suitable for intentional external and internal application to the patient excluding direct cardiac application. “Paddles” outside the box indicate the applied part is defibrillator proof.
[Medical Standard Definition:] F-type applied part (floating/isolated) complying with the specified requirements of EN 60601-1/UL 60601-1/CSA 601.1 Medical Standards to provide a higher degree of protection against electric shock than that provided by type B applied parts.
NOTE
The rating of protection against electric shock (indicated by symbol for CF or BF) is achieved only when used with patient applied parts recommended by GE.
TYPE B APPLIED PART: Non-isolated applied part suitable for intentional external and internal application to the patient excluding direct cardiac application.
[Medical Standard Definition:] Applied part complying with the specified requirements of EN 60601-1/UL 60601-1/CSA 601.1 Medical Standards to provide protection against electric shock, particularly regarding allowable leakage current.
1-14 |
Dash® 3000/4000/5000 |
2000966-338A |
Introduction
Fuse
Equipotential Stud: A ground wire from another device can be tied here to ensure the devices share a common reference point.
Alternating current (AC)
Power
Indicates where to press to open the writer door.
This symbol indicates that the waste of electrical and electronic equipment must not be disposed as unsorted municipal waste and must be collected separately. Please contact an authorized representative of the manufacturer for information concerning the decommissioning of your equipment.
Equipment Compliance Information
EN 60601-1 Component Classification
The following components meet EN 60601-1 classification standards.
2000966-338A |
Dash® 3000/4000/5000 |
1-15 |

Introduction
|
|
|
|
Degree of |
|
|
|
|
|
|
|
safety of |
|
|
|
|
Type of |
|
Degree of |
application in |
Method of |
|
|
|
|
protection |
the presence |
sterilization/ |
|
||
|
protection |
|
|
||||
|
Degree of protection against |
against |
of a flammable |
disinfection |
Mode of |
||
|
against |
||||||
|
electrical shock2 |
harmful |
anesthetic |
recommended |
Operation |
||
|
electrical |
||||||
|
shock1 |
|
ingress of |
mixture with |
by the |
|
|
|
|
water3 |
air or with |
manufacturer |
|
||
|
|
|
|
oxygen or |
|
|
|
|
|
|
|
nitrous oxide4 |
|
|
|
Monitor |
I |
BF (NBP, SpO2, CO2) |
|
|
|
|
|
|
CF (ECG, Resp, IP, Temp/CO) |
|
|
|
|
||
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
ICG Module |
N/A |
BF |
|
|
|
|
|
|
|
|
|
|
|
||
SAM Module |
B |
Ordinary |
Not Suitable |
N/A |
Continuous |
||
|
|||||||
|
|
|
|||||
PRN-50 Writer |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Remote Control |
N/A |
Not Marked |
|
|
|
|
|
|
|
|
|
|
|
||
TRAM-RAC 2A |
I |
|
|
|
|
|
|
(powered) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1The class of equipment — I or N/A (not applicable).
2The type of applied part — B, BF, CF, Not Marked or none (no applied parts).
3Ordinary equipment (enclosed equipment without protection against the ingress of water).
4Equipment not suitable for use in the presence of a flammable anesthetic mixture with air, oxygen or nitrous oxide.
Underwriters Laboratories, Inc. Classification
Medical Equipment
With respect to electric shock, fire and mechanical hazards only in accordance with
UL 60601-1 and CAN/CSA C22.2 NO.601.1.
AAMI EMI Environment Recommendations
Review the AAMI EMC Committee technical information report (TIR-18) titled Guidance of electromagnetic compatibility of medical devices for clinical/ biomedical engineers - Part 1: Radiated radio-frequency electromagnetic energy. This TIR provides a means to evaluate and manage the EMI environment in the hospital.
The following actions can be taken.
1-16 |
Dash® 3000/4000/5000 |
2000966-338A |
 Loading...
Loading...