3M 020SP User Manual

LifeASSURE™ 020SP Series
sterilizing grade filter cartridges and capsules
3M Purification Inc. pioneered the development of positively charge modified Nylon 6,6 filters for the pharmaceutical industry. LifeASSURETM 020SP series sterilizing grade filters and capsules are validated for absolute bacteria retention and provide reliable sterile filtration performance. In addition to a fixed bacteria retentive pore structure, LifeASSURE 020SP series membrane is charge modified to provide enhanced reduction of negatively charged biological contaminants such as endotoxin, virus and nucleic acid fragments. The combination of a validated bacteria retentive membrane, together with enhanced reduction of negatively charged contaminants, make LifeASSURE 020SP series membrane an ideal choice for pharmaceutical and biopharmaceutical sterilizing applications.
Each LifeASSURE 020SP series cartridge and capsule filter is supplied with a Certificate of Quality. A Validation Guide is available for ease of compliance with regulatory requirements. The Quality Management System is approved by an internationally recognized accrediting body to an ISO 9001:2008 Registered Quality System Standard.
Features & Benefits
Charge-modified Nylon 6,6 filter medium
•Retention of negatively-charged biological and particulate contaminants including endotoxins
•Inherently hydrophilic membrane for easy wet-out
High area, pleated membrane design
•High flow rates, low pressure drop
•Smaller filter systems, longer service life for reduced filtration costs
Reliable 0.2 µm validated performance
•Meets FDA definition of 0.2 µm sterilizing grade filter (107CFU/cm2 B. diminuta retention)
•Integrity test correlated with sterile filtration performance data and supported by Validation Guide
Robust cartridge construction
• Cartridges withstand mechanical and thermal stress including Steam In Place operations
Cartridges 100% integrity tested
• Ensures consistent performance to specifications, integrity testable pre-and post-use
ISO 9001:2008 - Registered Quality System
•Quality Management System approved by an accrediting body to an ISO 9001:2008 Quality Systems Standard
Tested and optimized for pharmaceutical and biological processing
•Materials of construction tested for biological safety (USP Class VI) and listed in Drug Master File (DMF) at FDA
Low extractable levels, no surfactants or adhesives used in manufacturing
• Minimal effect on filtrate quality and purity
Applications
•Large volume parenterals
•Biologicals
•Bioprocess-derived protein solutions
•Vaccines
•Diagnostics
•Purified water systems
•Solvents
•Ophthalmics
•Blood and serum fractions
•Cell culture media
•Reagents & buffers
•Pharmaceutical bulk chemical solutions
Optimized for Use with LifeASSURE™ PLA Series Prefilter Cartridge and Capsule Filters
LifeASSURETM 020SP Series sterilizing grade filters are designed for use with LifeASSURETM PLA series prefilters. Both filters feature Nylon 6,6 filter membrane. 3M Purification Inc.’s unique multi-zone micropourous membrane technology, used in LifeASSURE PLA series prefilters, provides superior life extension of LifeASSURE 020SP series final filters. Used together, LifeASSURE PLA series prefilters and LifeASSURE 020SP series final filters provide high system flow rates and safe, reliable microorganism retention.
For most applications, LifeASSURETM PLA045 series is the best first choice for prefiltration prior to LifeASSURE 020SP series sterilizing grade final filters. For applications where the particulate or colloidal level is extremely low and maximum final filter life is desired, choose LifeASSURETM PLA020 prefilters.
LifeASSURE 020SP Series Membrane Advantage
Absolute Bacterial Retention
LifeASSURE 020SP series filters are qualified for absolute retention of B. diminuta (ATCC 19146) following American Society of Testing and Materials (ASTM) methodology at a minimum challenge level of 107CFU/ cm2 of filter area. The results of bacteria challenge testing correlated
to non destructive integrity testing are available in 3M Purification Inc. LifeASSURETM Series 020SP Validation Guide (70-0201-8844-0).
Enhanced Retention of Negatively Charged Contaminants
LifeASSURE 020SP series Nylon 6,6 membrane is manufactured with positively charged functional groups. This feature provides enhanced retention of negatively charged contaminants including bacteria, endotoxins, viruses and nucleic acids.
Low Extractables and Broad Chemical Compatibility
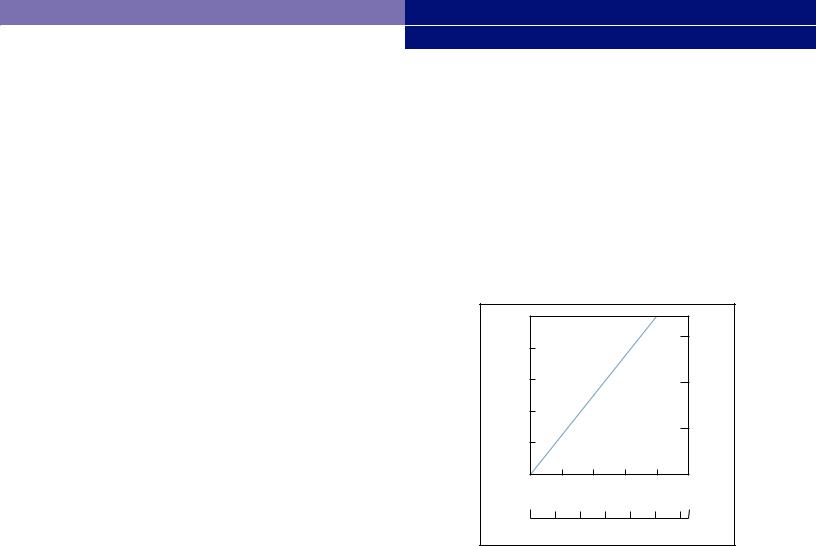
Graph 1. - LifeASSURETM 020SP Series 10” |
|
||||||
element water flow rates @ 77 °F (25 °C) |
|
|
|||||
|
5 |
|
|
|
|
|
|
(psid)PressureerentialDi |
4 |
|
|
|
|
0.3 |
(bar)PressureDierential |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
3 |
|
|
020SP |
|
0.2 |
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
0.1 |
|
|
1 |
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
0 |
1 |
2 |
3 |
4 |
5 |
|
|
|
|
Flow Rate (gpm) |
|
|
|
|
0 |
3 |
6 |
9 |
12 |
15 |
18 |
LifeASSURE 020SP series hydrophilic Nylon 6,6 filter membrane is inherently
low in extractables. Because the membrane is hydrophilic, surfactants are not required to achieve water wettability. The broad chemical compatibility of Nylon 6,6 membrane allows use with a wide range of process fluids.
High Cartridge and Capsule Flow Rates
LifeASSURE 020SP series cartridge and capsule filters offer high flow rates. Each configuration contains the highest possible pleat density without compromising access to the effective filtration surface area. Graph 1 shows the water flow rate and pressure drop characteristics of LifeASSURE 020SP series 10” cartridge and capsule filters.
Regulatory Compliance & Quality Assurance
LifeASSURE 020SP series sterilizing grade filters are 100% integrity tested during manufacture to ensure that they are integral. LifeASSURE 020SP series filters are manufactured in a controlled environment using an ISO 9001:2008 Registered Quality System. An identification system permits traceability of all raw material components. All materials of construction are 21 CFR listed and have passed USP Class VI Biological Test for Plastics at 121 °C. A Certificate of Quality is included with every filter certifying quality attributes and lot release testing. Representative sample filters from each manufacturing lot are tested for microbial retention, endotoxin, oxidizable substances, and forward flow integrity
LifeASSURE 020SP series sterilizing grade filters are qualified for absolute retention of B. diminuta (ATCC 19146) following ASTM methodology at a minimum challenge level of 107CFU/cm2. LifeASSURE 020SP series filters meet the definition of a sterilizing grade filter as described in the “FDA Guideline on Sterile Drug Products Produced by Aseptic Processing” (September 2008) and are manufactured in accordance with 3M Purification Inc.’s active Drug Master File (DMF).
2
 Loading...
Loading...